Original Article
, Volume: 12( 9)Influence of Some Brown Seaweed Extracts on Germination and Cytological Responses of Trigonella foenum-graecum L.
- *Correspondence:
- El-Sheekh MM, Botany Department, Faculty of Science, Tanta University, 31527 Tanta, Egypt, Email: mostafaelsheikh@science.tanta.edu.eg
Received: July 07, 2016; Accepted: July 12, 2016; Published: July 18, 2016
Citation: El-Sheekh MM, Ismail MM, Hamouda MM. Influence of Some Brown Seaweed Extracts on Germination and Cytological Responses of Trigonella foenum-graecum L. Biotechnol Ind J. 2016;12(9):104.
Abstract
The objective of this study was to evaluate the effects of the brown algae Sargassum vulgare, Colpomenia sinuosa and Padina pavonica extracts on the germination rate, cell division and protein pattern of Trigonella foenum-graecum L (fenugreek). The seaweed extracts elicited better response at lower concentrations while higher concentrations decreased the germination rate of fenugreek seeds when compared to control. With respect to protein pattern of fenugreek, 20 bands were recorded, nine monomorphic, eight polymorphic and three unique. Some bands are specific to each seaweed which may be attributed to their chemical composition. Extract of S. vulgare was more effective on germination of fenugreek seeds than the other seaweeds extracts used in this investigation. These results suggest that cytotoxic effect of high concentration of seaweeds extract may be due to accumulation of their phenolic content. The results of the present investigation also suggest that seaweeds can be exploited for stimulation the growth of economic crops instead of chemical fertilizers.
Keywords
Brown seaweed extract; Biofertilizer; Cytological aberration; Germination; Protein profile; Trigonella sp.
Introduction
Fenugreek (Trigonella foenum-graecum L.) is one of the condiments known to mankind and has been cultivated for a very long time. It has been popularly used as food as well as medicine in many countries such as Egypt, India, Pakistan and Sudan [1]. Fenugreek seeds contain 26% protein, 5.8% fat and 44.1% carbohydrate [2]. Beside that it has medical properties such as role of phyto-estrogens, diosgenins to fight breast cancer, reduction of serum cholesterol [3].
Seaweeds or marine macroalgae are the primitive group of organisms with no true roots, stems and leaves, and they are one of the important marine living resources with tremendous commercial importance [4]. They are the oldest family of plants on earth and have admirable qualities of being flexible, tenacious and prolific [5]. They are classified by researchers as the most important group of organisms which can be widely used in plants nutrition [6].
Seaweed extracts are known to enhance seeds germination, seedlings development and increase plant tolerance to environmental stresses [7,8]. Seaweeds are one of the best biofertilizer for the reason that they contain polysaccharide content [9] and an adequate amount of potassium, nitrogen, growth promoting hormones, in addition to micronutrients make it as excellent fertilizer [10]. Unlike chemical fertilizer, they are biodegradable, non-polluting and nontoxic to human, animals and birds. Natural seaweed as fertilizer substituting conventional synthetic fertilizer [11] which have degraded the fertility of soil making it acidic and rendering it unsuitable for rising crops [12].
Seaweed extracts contains naturally occurring substances that play an important role in cell division, cell elongation, cell differentiation factors and the synthesis of protein [13]. Seaweed composition may vary even in the same species, under different environments, causing different sets of protein expression [14]. Shepard et al. [15] stated that there are different factors impacts on protein profile of organisms e.g., development stages, internal and external conditions.
In modern agriculture, marine macroalgae were used as organic fertilizers for many crops [16-20], hence, brown and red algae contain high amount of potassium but have low nitrogen and phosphorus [21]. Ramya et al. [22] concluded that brown alga which contains more hormones, nutrients, vitamins etc., than other seaweeds could be effective in enhancing the growth and physiology of certain plants.
This investigation aimed to assess the influence of different concentrations of marine algae Sargassum vulgare, Colpomenia sinuosa and Padina pavonica extracts on germination, rate of cell activity (mitotic index) with their chromosomal aberration assay and protein pattern of Fenugreek.
Materials and Methods
Collection and identification of seaweeds
The marine brown seaweeds used for this study were freshly collected from Rocky Bay of Abu Qir (longitudes 30°05'- 30°22' E and latitudes 31°16'- 31°21' N.), Alexandria, Egypt during Spring 2014 (Figure 1). The collected seaweeds were brought to the laboratory in plastic bags containing seawater to prevent evaporation. Seaweeds were cleaned from epiphytes and rock debris and were given a quick freshwater rinse to remove surface salts. Subsequently the seaweeds were processed as herbarium specimens on the same day of collection and deposited in Taxonomy Museum Marine Environmental Division (National Institute of Oceanography and Fisheries, Kayet Bay, Alexandria, 21556, Egypt); others were preserved in 5% formalin in seawater for taxonomic classification. Other cleaned seaweeds were air dried in the shade at 40°C for several days. The dried samples were finely powdered and stored at 20°C until use. All seaweeds were identified following the previous methods [23-26]. The names of the species were used according to Guiry and Guiry [27].
Preparation of seaweed liquid extracts (SLE)
Dried seaweeds were heated with sterile distilled water in a ratio 25:100 (w/v) at 60°C for 45 min. Then the extracts were filtered through a filter paper [28] and stored at 4°C for further experimental studies. Different concentrations of SLE (5%, 10%, 15%, 20% and 25%) were prepared by diluting extracts with distilled water.
Chemical analyses of seaweed liquid extracts
Macro and micro elements such as "sodium, potassium, cadmium, zinc, manganese and copper" concentrations were determined by reading the absorbance in the atomic absorption spectrophotometer AA-6800 and total nitrogen was carried out with Micro-Kjeldahel method [29]. Carbohydrate content was estimated according to Dubois et al. [30] and phenol was estimated according to the method described by Lim et al. [31].
Plant materials
The seeds of Fenugreek (Leguminosae) were kindly provided by the Agriculture Research Centre (ARC), Giza, Egypt. The seeds with uniform size, color and weight were chosen for the experimental purpose and surface sterilized with 0.5% NaCl2 for 1 min and thoroughly washed by sterilized distilled water. Ten seeds were soaked for each concentration of SLE (5%, 10%, 15%, 20% and 25%) for 12 h. Control seeds were soaked in tap water. Seeds were then placed on sterilized Petri dishes 9 cm containing Whatman No. 1 filter paper at room temperature (28°C ± 1°C). The filter paper was kept moist by regular addition of tap water for control seeds and treatment seeds. The germination percentage was recorded on 7th day after sowing.
Cytological studies
To assess the effects of seaweed extracts on cell division and chromosomes, at least ten roots of ten different seedlings from each treatment were fixed in freshly prepared fixative composed of absolute ethanol and glacial acetic acid (3:1) for 24 h and kept in 70% ethanol at 4°C until use for cytological preparations as Feulgen's squash technique to calculate the mitotic index (dividing cell frequency) and % chromosomal abnormalities [32].
Protein profiling
The pre-soaked seeds of Trigonella in seaweed extracts and water as control were weighted and homogenized, respectively, in liquid nitrogen with pre-frozen mortar and pestle. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDSPAGE) 12% was used to resolve the protein pattern of seeds extracts. Pre-soaked seeds and control plants firstly washed with distilled water then the protein was extracted from 0.01 g of seeds flour using 400 μl of extraction buffer that contained 0.05 m Tris-HCL pH 8.0, 0.2% SDS, SM urea and 1% mercapto ethanol. Seeds flour was thoroughly mixed with buffer by vortex. The supernatant of each sample was collected and transferred to a new tube after centrifugation at 15,000 rpm for 10 min. The supernatants were then boiled at 95°C for 5 min. Electrophoresis was carried out in a discontinuous SDS-PAGE as described by Laemmli [33] using acrylamide gel. The gel was then photographed with digital camera and the presence or absence of bands was scored as 1 or 0, respectively.
Total soluble protein
Total soluble protein was estimated by the method of Bradford [34].
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed by Student–Newman–Keuls (SNK) test and differences at P< 0.0001 were considered as significant. Molecular weights of protein bands were calculated using Lab Image software version 2.7 produced by Kapelan GmbH, Germany.
Results
Taxonomic description of collected brown seaweeds
The collected species were identified as presented in the literatures, checked for synonyms and latest accepted names, referred to its systematic groups and described. The collected species were S. vulgare Agardh, P. pavonica (Linnaeus) Thivy and C. sinuosa (Martens ex Roth) Derbes et Solier from Phaeophyceae.
Phylum: Phaeophyta
Class: Phaeophyceae
Order (1): Fucales
Family: Sargassaceae
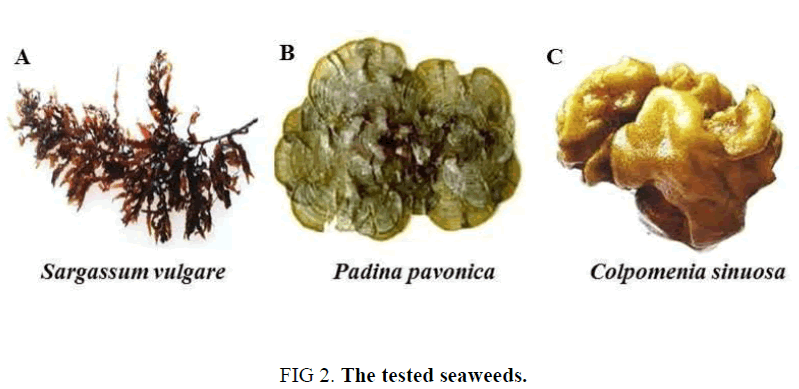
Genus: Sargassum vulgare Agardh (Figure 2A)
Description: Thalli are dark brown in color. Fronds are pyramidical in shape; they reach about 20 cm to 25 cm in length. The stripe is small and cylindrical; it is attached to the substratum by a small disk and gives rise from the distal end to caespitose primary branches. Leaves are large, linear or serrate, with dentate or undulate margin, reach 4 cm tall and spirally arranged. There are one or more air bladders which are be modified from basal members of the axillary branch.
Remarks: Thallus forms a distinct community and associate with Jania rubens. Present of zooplankton eggs on the leaves especially lower leaves and also a lot of bivalve species.
Order (2): Dictyotales
Family: Dictyotaceae
Genus: Padina pavonica (Linnaeus) Lamouroux (Figure 2B)
Description: Thallus is light to dark brown in color, membranous, fan shaped, narrow rounded stalk, up to 15 cm tall, 12 cm in breadth, margin undulate, hairs developing all over the thallus as concentric zones, basal portion forming rhizomatous discs.
Remarks: Jaina rubens epiphytic on it.
Order (3): Ectocarpales
Family: Scytosiphonaceae
Genus: Colpomenia sinuosa (Martens and Roth) Derbes and Solier (Figure 2C)
Description: Thallus is greenish brown in color, 5cm to 10 cm in diameter, seems to be a ball like structure, sessile, globular, hollow, forming air filled and irregularly lobed vesicles with corrugated, smooth surface, relatively thick walled and attached to the substrate with creeping filaments.
Remarks: Small and young thalli are the most common and their color slime green.
Chemical analysis of different seaweed extracts
The chemical properties of different seaweed extracts were analyzed (Table 1). The seaweed extract of S. vulgare contained higher levels of carbohydrates 234.87 and nitrogen 9.77 (mg/g DW) and lower levels of phenol 0.165 as compared to the other two algal extracts. S. vulgare contained the lowest level of Mn (0.337 ppm), Na (0.0219 ppm) and Cu (0.054 ppm), while it contained higher level of Zn (2.08 ppm) as compared with the other two extracts. On the other hand, the lowest level of Cd (0.014 ppm) was recorded in the extract of P. pavonica (Table 2).
| Species | Conc. (mg/g DW) | ||
|---|---|---|---|
| Phenol | Carbohydrates | Nitrogen | |
| Sargassum vulgare | 0.175 ± 0.010 | 234.870 ± 0.011 | 9.77 ± 4.011 |
| Colpomenia sinuosa | 0.220 ± 0.020 | 30.737 ± 0.058 | 3.351 ± 2.215 |
| Padina pavonica | 0.260 ± 0.010 | 103.926 ± 0.126 | 9.233 ± 1.520 |
Table 1. The total phenol, carbohydrate and nitrogen (mg/g DW) contents of the tested brown seaweeds. Data are mean of three replicates (± SD).
| Species | Conc. (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| N | Na | K | Cu | Cd | Zn | Mn | |
| Sargassum vulgare | 9.77 ± 4.011 | 0.022 ± 0.002 | 0.013 ± 0.001 | 0.054 ± 0.006 | 0.004 ± 0.001 | 2.080 ± 0.009 | 0.337 ± 0.008 |
| Colpomenia sinuosa | 3.351 ± 2.215 | 0.025 ± 0.008 | 0.008 ± 0.000 | 0.075 ± 0.004 | 0.002 ± 0.000 | 0.832 ± 0.011 | 0.811 ± 0.009 |
| Padina pavonica | 9.233 ± 1.520 | 0.024 ± 0.005 | 0.011 ± 0.002 | 0.061 ± 0.009 | 0.006 ± 0.001 | 0.291 ± 0.009 | 0.735 ± 0.010 |
Table 2. The concentration of some macro and micro elements in the tested brown seaweeds (ppm). Data are mean of three replicates (± SD).
Seed germination
The effect of different seaweed extracts on germination percentage of Fenugreek seeds ranged from 31% to 87.3% according to brown seaweeds species and concentrations used (Table 3). The seaweed extracts treatment increased the germination percentage significantly when compared to the control. The low concentration of the tested brown seaweeds extract showed high germination rate as compared with higher concentration. The highest seed germination was found in S. vulgare, followed by C. sinuosa then P. povonica treatments. and the lowest rate of germination was determined in P. povonica treatments as compared with other treatments.
| Species Concentration (%) | Control | Germination (%) | Protein (mg/g) |
| 71 | 169.889 | ||
| Sargassum vulgare | 5 | 87.33** | 219.217 ± 1.253** |
| 1 0 | 82.67** | 205.579 ± 2.450** | |
| 15 | 77.00** | 202.388 ± 3.015** | |
| 20 | 64.67** | 196.149 ± 2.105** | |
| 25 | 61.00** | 189.475 ± 1.548** | |
| F | 129.144 | 4.182 | |
| Colpomenia sinuosa | 5 | 76.33** | 210.077 ± 0.045** |
| 1 0 | 70.33 | 209.642 ± 1.245** | |
| 15 | 65.00** | 198.761 ± 1.897** | |
| 20 | 50.00** | 191.071 ± 2.541** | |
| 25 | 31.00** | 185.262 ± 2.452** | |
| F | 87.353 | 7.084 | |
| Padina pavonica | 5 | 82.00** | 212.977 ± 2.545** |
| 1 0 | 78.67** | 211.528 ± 3.458** | |
| 15 | 73.00* | 205.434 ± 0.524** | |
| 20 | 61.00** | 188.315 ± 1.985** | |
| 25 | 35.00** | 188.025 ± 0.975** | |
| F | 125.038 | 5.570 |
Table 3. The Effect of different concentrations of the tested brown seaweed extracts on the seed germination percentage and protein content (mg/g dry wt) of Fenugreek. Data are mean of three replicates (± SD). *Significant at p < 0.0001 level
Protein content
The increase in the protein content of pre-soaked seeds was directly proportional to the decrease in the concentration of the tested seaweed extracts according to each type of algae. The highest protein content of Fenugreek was recorded by S. vulgare as compared to the other two tested seaweeds (Table 3).
Cytological changes
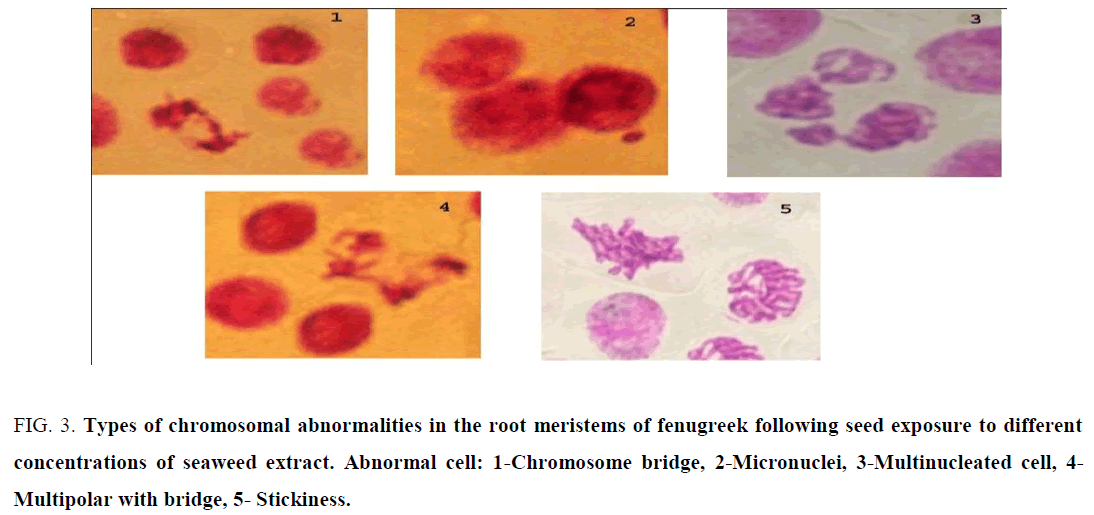
For cytological study, mitotic activity of seeds treated with different species of brown seaweed extracts was highly significant as compared to control. Mitotic index was recorded to be 18.1% in control set. The highest mitotic index 33.5% was recorded in S. vulgare extract treatment, followed by C. sinuosa 30.9% and 25% was recorded in P. povonica. However, a gradual reduction in mitotic index (from 33.5% to 27.8% in S. vulgare, 30.9 to 13.7 % in C. sinuosa and 25% to 14.9% in P. povonica) was recorded with the increasing the concentration of seaweed extract in Fenugreek plant (Table 4). The abnormality indexes were high with the concentration 25% of both C. sinuosa and P. povonica. Chromosomal bridge, micronuclei, multinucleated cell, multipolar cell and stickiness were recorded with the highly concentration extract of all treatments (Figure 3).
| Macroalgae spp. |
Conc.(%) | TotalExaminedcell | Total mitoses |
Non dividing cell | Dividing cell | Mitotic index (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| InterphaseIndex(%) | Prophase index(%) | Metaphase Index (%) |
AnaphaseIndex(%) | TelophaseIndex (%) | Abnormality Index (%) |
|||||
| S. vulgare | 0 | 1105 | 200 | 81.9 | 55.0 | 20.0 | 15.0 | 10.0 | - | 18.1 |
| 5 | 1053 | 354 | 66.3 | 56.5 | 22. 3 | 11.9 | 9.3 0 | - | 33.6 | |
| 10 | 1063 | 337 | 68.3 | 41.2 | 24.9 | 18.7 | 15.2 | - | 31.7 | |
| 15 | 840 | 245 | 70.8 | 37.5 | 26.1 | 22.9 | 13.5 | - | 29.2 | |
| 20 | 1000 | 282 | 71.8 | 56.7 | 17.7 | 14.3 | 11.3 | - | 28.2 | |
| 25 | 863 | 240 | 72.2 | 66.7 | 16.7 | 10.8 | 5.8 0 | - | 27.8 | |
| C. sinuosa | 5 | 976 | 300 | 69.3 | 60.0 | 20.0 | 11.3 | 8.7 0 | - | 30.7 |
| 10 | 984 | 269 | 72.6 | 46.1 | 22.3 | 19.7 | 11.9 | - | 27.3 | |
| 15 | 942 | 223 | 76.3 | 49.8 | 23.8 | 18.8 | 7.60 | - | 23.7 | |
| 20 | 762 | 156 | 79.5 | 59.6 | 19.8 | 12.8 | 7.70 | - | 20.5 | |
| 25 | 673 | 93 | 86.2 | 32.2 | 23.6 | 11.8 | 9.60 | 22.8 | 13.8 | |
| P. pavonica | 5 | 1000 | 250 | 75.0 | 60.0 | 20.0 | 12.0 | 8.00 | 25.0 | |
| 10 | 996 | 235 | 76.4 | 54.9 | 18.7 | 15.3 | 11.1 | 23.6 | ||
| 15 | 1240 | 262 | 78.8 | 46.9 | 26.7 | 21.5 | 4.90 | 21.2 | ||
| 20 | 1200 | 246 | 79.5 | 48.8 | 24.4 | 16.3 | 10.5 | 20.5 | ||
| 25 | 836 | 125 | 84.7 | 38.4 | 17.6 | 12.0 | 8.00 | 24.0 | 14.9 | |
Table 4. Number of cells examined and percentage of normal and abnormal cells at the stages of mitoses and the value of mitotic index in roots of Fenugreek subjected to different concentrations of brown seaweed aqueous extracts.
Figure 3: Types of chromosomal abnormalities in the root meristems of fenugreek following seed exposure to different concentrations of seaweed extract. Abnormal cell: 1-Chromosome bridge, 2-Micronuclei, 3-Multinucleated cell, 4- Multipolar with bridge, 5- Stickiness.
For SDS-polyacrylamide gel (Table 5) illustrates the banding pattern of seed protein polypeptides for the fenugreek seeds plant. In total, 20 protein bands have been observed; 8 bands are polymorphic, 9 are monomorphic and 3 unique. The molecular weight as well as presence or absence of bands in every treatment has been scored in Table 5. The profiles of protein pattern for plant treated with seaweed extract differ from the control profile. The molecular weight of the bands ranged from 210 KDa to 15 KDa. Some bands were specific for certain genotypes of algae; for example the bands with MW of 131 and 123 KDa were specific for C. sinuosa, 111 and 106 KDa were specific for P. povonica and the band with MW 182 KDa was specific for S. vulgare. The band with MW 142 KDa was recorded in both C. sinuosa and P. povonica that correlated with the high concentration extract (25%).
| Band number | M.W | Spp. | Sargassum vulgare | Colpomenia sinuosa | Padina pavonica | Band type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont.% | 5 | 10 | 15 | 20 | 25 | 5 | 10 | 15 | 20 | 25 | 5 | 10 | 15 | 20 | 25 | |||
| 01 | 210 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 02 | 182 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P |
| 03 | 162 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 04 | 142 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | P |
| 05 | 138 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | U |
| 06 | 131 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | P |
| 07 | 123 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | P |
| 08 | 111 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | P |
| 09 | 106 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | P |
| 10 | 100 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | U |
| 11 | 89 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 12 | 70 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | P |
| 13 | 64 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 14 | 45 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 15 | 40 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | P |
| 16 | 38 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 17 | 31 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 18 | 22 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | U |
| 19 | 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
| 20 | 15 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | M |
Table 5. Molecular weight (M.W.) and presence (1) or absence (0) scores for the seedling proteins in fenugreek subjected to different concentrations of brown seaweed extracts.
Discussion
Application of seaweed as biofertilizer will be useful in enriching the soil and achieving higher production in the place of costly chemical fertilizer. Significant differences were observed in the germination rate of fenugreek at lower concentrations than higher concentrations of the tested brown seaweeds and this is may be due to the presence of growth promoting substances such as indole-3-acetic acid (IAA) and indole butyric acid (IBA), gibberellins A and B, cytokinins, micronutrients (Fe, Cu, Zn, Co, Mo, Mn and Ni) [35]. The present results are in agreement with Sivasankari et al. [36] who recorded that the low concentration of aqueous extracts of Sargassum wightii promoted the seedling growth of Vigna sinensis. Sivakumar and Gandhi [37] stated that the increased growth parameters of V. mungo at lower concentration may be due to the presence of higher levels of N, P, K in the seaweed extract of S. wightii. Kumar et al. [38] found that the dilute seaweed extracts more effective than the concentrated extract. John and Yuvaraj [39] reported that the seed germination, shoot length and root length of V. radiate (L.) were maximum at 10% SLF of Colpomenia sinuosa.
Protein content of fenugreek seeds was increased by decrease the concentration of seaweed extracts. The negative responses with increase the concentration of seaweed extracts on seed germination and protein content can be attributed to presence of regulator hormones or high levels of minerals that inhibited the growth [40]. In the present study, S. vulgare was the effective seaweed extract on fenugreek plant which showed higher germination rate and protein content and this may be due to that S. vulgare contains low amount of phenol and high amount of carbohydrate and nitrogen which play a significant role in crop quality [41] and also that the activation of nitrate reductase, key enzyme of nitrogen metabolism which enhanced the growth [42]. Our findings coincide with Taylor and Wilkinson [43] who reported that the growth enhancing potential of seaweed might be attributed to the presence of carbohydrate, phenyl acetic acid. Pise and Sabale [44] showed that the extracts obtained from S. ilicifolium stimulated shoots growth, carbohydrates, proteins, free amino acids, polyphenols, nitrogen content and increase mass of fenugreek.
The data revealed that the rate of mitotic activity in fenugreek subjected to seaweed extracts were highly significant compared to control, this may be due to seaweed extracts being rich in macro and micro elements, important plant hormones like Auxins, Gibberellins and Cytokinin which induce cell division and increasing cell enlargement [45,46]. The rate of mitotic index in treated plant decreased with the higher concentration of seaweed extracts with the high concentrations of Ca, K, Mg, Na, Cu, Fe, I, and Zn [47] that make reduction in cell division and they may contain different levels of minerals, biostimulants are unable to provide all the nutrients needed by a plant in required quantities [48]. Some abnormalities of chromosome appeared in the fenugreek plant treated with higher concentration of seaweed extract of C. sinuosa and P. povonica, these two algae recorded the higher amount of phenol, that causes increase in the formation of reactive oxygen species (ROS), which, in excess, cause damage to the membranes (lipid peroxidation), protein oxidation and DNA fragmentation [49]. These results are in agreement with few studies that have investigated the toxic effects of brown seaweed extract that could generate (ROS), which can damage DNA causes chromosomal aberration [50]. The decreased mitotic index is attributed by the formation of aberrant cells and the result is similar with the same observation in the same crop fenugreek with heavy metals stress [51].
SDS-polyacrylamide gel protein profiles of Fenugreek treated with seaweed extracts showed additional protein bands compared to the control plants. This is may be due to marine algae provide an excellent source of bioactive compounds such as essential fatty acids, vitamins, amino acids, minerals, and growth promoting substances [52], which act either by turning on the expression of certain genes or by being involved in the modification of key gene products and induced proteins [53,54]. Also, Johri and Mitra [13] stated that theses substance from seaweeds play a significant role in cell division, cell elongation and cell differentiation factors and cell type specific gene. The changes of gene expression on biofertilizer treatment may be to differnce in amount of nitrogen and carbohydrate that changed the metabolic activites that assiociated with protein change and nucleic acids in plants [55]. New band with MW 182 KDa correlated with the higher concentration of C. sinuosa and P. povonica this may be due to the high amount of phenol which including antioxidant properties at higher concentration these antioxidants can damage essential bio-molecules: proteins, DNA and lipids and caused various change in polypeptide and leading to accelerated ageing and subsequent senescence [56]. The heavy metals content cause damage to the physiological process in the cell and biomolecules such as membrane lipid, proteins, enzymes, nucleic acid among others [57], and also cause oxidative damage to plants either directly or indirectly by triggering an increased level of production of reactive oxygen species (ROS) which include superoxide radical (O-), hydroxyl radical (OH+) and hydrogen peroxide (H2O2) [58].
Conclusion
From the above results, it is suggested that the brown seaweeds liqiuid fertilizer (BSLF) from the tested brown seaweeds can be used at low concentrations for enhancing the seed germination, biochemical constituents and histology of Trigonella foenum-graecum L (fenugreek) seeds. This observation is further supported by the fact that the performance of BSLF is eco-friendly, easily manageable input farming and a self-regenerating system which provide nutrients and maintains health status. Hence the use of modern agriculture in conjunction with traditional farming practices is the sustainable solution for the future.
Acknowledgments
Authors are thankful to Prof. Abd El-Ghani Khalil, Oceanography Dept., Faculty of Science, Alexandria University, Egypt for confirming the taxonomical description and giving us valuable and useful suggestions.
References
- Mohiuddin S, Roshan AO, Zamir A, et al. Laboratory evaluation of some vegetable oils as protectants of tored products. Pak J Sci Indus Res. 1993;36:377-9.
- Patil SP, Niphadkar PV, Bapat MM. Allergy to fenugreek (Trigonella foenum graecum). Ann Allergy Asthma Immunol. 1997;78(3):297-300.
- Amin A, Alkaabi A, Al-Falasi S, et al. Chemopreventive activities of Trigonella foenum graecum (Fenugreek) against breast cancer. Cell Biol Int. 2005;29(8):687-94.
- Kaliaperumal N, Chennubhotla VSK, Kalimuthu S. Seaweed resources of India. CMFRI Bulletin. 1987;41:51-4.
- John PPJ, Patric RD. Seasonal variability of some Enteromorpha species in Kanyakumari region, the southern coast of Tamil Nadu. Asian J Biol Life Sci. 2012;1:147.
- Tuhy L, Chowanska J, Chojnacka K. Seaweed extracts as biostimulants of plant growth: review. Chemik. 2013;67(7):636-41.
- Zhang XZ, Ervin EH. Cytokinin-Containing Seaweed and Humic Acid Extracts Associated with Creeping Bentgrass Leaf Cytokinins and Drought Resistance. Crop Sci. 2004;44(5):1737-45.
- Zhang X, Ervin EH. Impact of Seaweed Extract-Based Cytokinins and Zeatin Riboside on Creeping Bentgrass Heat Tolerance. Crop Sci. 2008;48(1):364-70.
- Stephenson WA. Seaweeds in agriculture and horticulture. 3rd ed. California: Ratequer Peruma Valley Ition; 1974. p. 241.
- Mishra DJ, Rajvir S, Mishra UK, et al. Role of bio-fertilizer in organic agriculture: A Review. J Recent Sci. 2013;2:39-41.
- Hong DD, Hien HM, Son PN. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J Appl Phycol. 2007;19:817-26.
- Mabeau S, Fleurence J. seaweed in food products: biochemical and nutritional aspects. Trend Food Sci Technol. 1993;4:103-7.
- Johri MM, Mitra D. Action of plant hormones. Curr Sci. 2001;80(2):199-205.
- Contreras L, Moenne A, Gaillard F, et al. Proteomic analysis and identification of copper stress-regulated proteins in the marine alga Scytosiphon gracilis (Phaeophyceae). Aquat Toxicol. 2010;96(2):85-9.
- Shepard JL, Olsson B, Tedengren M, et al. Protein expression signatures identified in Mytilus edulis exposed to PCBs, copper and salinity stress. Mar Environ Res. 2000;50(1-5):337-40.
- Rao RK. Effect of aqueous seaweed extract on Zizyphus mauritiana Lam. J Ind Bot Soc. 1991;71:19-21.
- Lopez-Musquera, ME, Pazas P. Effect of seaweed on potato yield and soil chemistry. Biol Agricul Horticul. 1997;14:199-205.
- Rajarajan R. Thesis. University of Madras. 2002.
- Jothinayagi N, Anbazhagan C. Effect of seaweed liquid fertilizer of Sargassum wightii on growth and biochemical characteristics of Abelmoschus esculentus(L.). Recent Res Sci Technol. 2009;1:155-8.
- Zodape ST, Mukherjee S, Reddy MP, et al. Effect of Kappaphycus alvarezii (Doty) Doty ex silva. extract on grain quality, yield and some yield components of wheat (Triticum aestivum L.). Int J Plant Prod. 2009;3(2):97-101.
- Waaland JR. Commercial utilization in the biology of seaweeds. In: LobbanCS, Wynne MJ, editors. The Biology of Seaweeds. Oxford: Blackwell Scientific; 1981. p. 726-41.
- Ramya SS, Nagaraj S, Vijayanand N. Biofertilizing efficiencyofbrownandgreenalgaeongrowth,biochemicaland yield parameters of Cyamopsis tetragonolaba (L.) Taub. Recent Res Sci Technol. 2010;2(5):45-52.
- Taylor WR. Marine algae of tropical and subtropical Americas. University of Michigan Press. Ann Arbor. 1960;870.
- Abbott IA, Hollenberg IG. Marine algae of California. California: Stanford University Press; 1976. p. 827.
- Aleem AA. The marine Algae of Alexandria, Egypt.Egyptian Books House: Egypt;1993.
- Jha B, Reddy CRK, Thakur MC, et al. Seaweeds of India the Diversity and Distribution of Seaweeds of the Gujarat Coast. New York: Springer; 2009.
- Guiry MD, Guiry GM, Algae Base. World-wide electronic publication. National University of Ireland, Galway;2011. Available from: http://www.algaebase.org/
- Rathore SS, Chaudhary DR, Boricha GN, et al. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S Afr J Bot. 2009;75(2):351-5.
- https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf
- Dubois M, Gillies KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350-6.
- Lim SN, Cheung PC, Ooi VE, et al. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J Agric Food Chem. 2002;50(13):3862-6.
- Pienaari DEV. J South Afr Bot. 1955;21:1.
- Laemmli UK. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680-5.
- Asryants RA, Duszenkova IV, Nagradova NK. Determination of Sepharose-bound protein with Coomassie brilliant blue G-250. Anal Biochem. 1985;151(2):571-4.
- Kalaivanan C, Venkatesalu V. Utilization of seaweed Sargassum myriocystum extracts as a stimulant of seedlings of Vigna mungo (L.) Hepper. Span J Agricul Res. 2012;10(2):466-70.
- Sivasankari S, Venkatesalu V, Anantharaj M, et al. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour Technol. 2006;97(14):1745-51.
- Sivakumar K, GandhiA.Potentiality of Sargassum wightii as a fertilizer on Black gram and their growth and yield by image analysis.Seaweed ResUtiln. 2010;32(1&2):75-83.
- Kumar NA, Vanlalzarzova B, Sridhar S, et al. Effect of liquid seaweed fertilizer of Sargassum wightii grev. on the growth and biochemical content of green gram (Vigna radiate (L.)R. wilczek). Recent Res Sci Technol. 2012;4:40-5.
- John PP, Yuvaraj P. Effect of Seaweed Liquid Fertilizer of Colpomenia sinuosa (Mert. ex Roth) Derbes & Solier (Brown Seaweed) on Vigna radiata (L.) R. Wilczek. In Koothankuzhi, Tirunelveli district, Tamil Nadu, India. Ind Int J Pure Appl Biosci. 2014;2(3):177-84.
- Erulan V, Soundrapandian P, Thirumaran G, et al. Studies on the Effect of Sargassum polycystum (C.agardh, 1824) Extract on the Growth and Biochemical Composition of Cajanus cajan (L.) Mill sp. Amer Euras J Agricul Environ Sci. 2009;6(4):392-9.
- Sisson VA, Rufty TW, Williamson RE. Nitrogen-Use Efficiency among Flue-Cured Tobacco Genotypes. Crop Sci. 1991;31(6):1615-20.
- Latique S, Chernane H, Mansori M, et al. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgarisvariety Paulista) under hydroponic system. Europ Sci J. 2013;9(30):174-91.
- Taylor IEP, Wilkinson AJ. The Occurrence of gibberellins - like substance in algae. Int Phycol Soc. 1977;16:37-42.
- Pise NM, Sabale AB. Effect of Seaweed Concentrates on the Growth and Biochemical Constituents of Trigonella Foenum-Graecum L. Phytol J. 2010;2(4):50-6.
- Gollan JR, Wright JT. Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Fresh Water Res. 2006;57:685-94.
- SarhanTZ. Horticulture Sciences and Landscape Design (Vegetable). University of Mosul, College of Agriculture and Forestry, 2008.
- MacArtain P, Gill C, Brooks M, et al. Nutritional value of edible seaweeds. Nutr Rev. 2007;65(12 Pt 1):535-43.
- Schmidt RE, Ervin EH, Zhang X. Questions and answers about biostimulants. Golf Course Manag. 2003;71(6):91-4.
- Mishra P, Bhoomika K, Dubey RS. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma. 2013;250(1):3-19.
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727-41.
- Patel KP, Patel KM. Cytological changes in Trigonella Foenum-graecum (l.) Under the Cadmium Stress. J Life Sci Technol. 2013;1(1):10-3.
- Yokoya NS, Stirk WA, Staden J, et al. Endogenous Cytokinins, Auxins, and Abscisic Acid in Red Algae from Brazil. J Phycol. 2010;46:1198-1205.
- Liu W, Hildebrand DF, Grayburn WS, et al. Effects of exogenous auxins on expression of lipoxygenases in cultured soybean embryos. Plant Physiol. 1991;97(3):969-76.
- Sridhar S, Rengasamy R. Potential of seaweed liquid fertilizers (SLFs) on some agricultural crop with special reference to protein profile of seedlings. Int J Develop. 2011;1:55-7.
- Sanchez E, Soto JM, Nunez A, et al. Biosynthesis of non-structural carbohydrates and their distribution in greenbean plants (phaseolus vulgaris l. Cv. Strike): deficiency vs. toxicity of nitrogen. Rev Fitotecnia Mex. 2005;28(1):55-61.
- Peerapornpisal Y, Amornlerdpisn D, Jamjai U, et al. Antioxidant and Anti-inflammatory Activities of Brown Marine Alga, Padina minor Yamada. Chiang Mai J Sci. 2010;37(3):507-16.
- Mishra RK, Singhal GS. Function of Photosynthetic Apparatus of Intact Wheat Leaves under High Light and Heat Stress and Its Relationship with Peroxidation of Thylakoid Lipids. Plant Physiol. 1992;98(1):1-6.
- Smiri M, Jalali N, Ghoul EJ. Cadmium affects the thioredoxin reductase/ thioredoxin system in germinating pea seeds. J Plant Int. 2013;8:125-133.