Short communication
, Volume: 14( 3)Industrially Viable Demethylation Reaction in Synthesis of Raloxifene Hydrochloride
- *Correspondence:
- Chavakula R , Research and Development, Aurobindo Pharma Limited, Survey No: 71 and 72, Indrakaran Village, Sangareddy Mandal, Medak Dist-502329, Telangna State, India, Tel: +91 40 6672 5000; E-Mail: ramadas.chavakula@gmail.com
Received: September 03, 2018; Accepted: September 11, 2018; Published: September 20, 2018
Citation: Chavakula R, Saladi CJS, Mutyala NR et al. Industrially Viable Demethylation Reaction in Synthesis of Raloxifene Hydrochloride. Org Chem Ind J. 2018;14(3):128
Abstract
Introduction
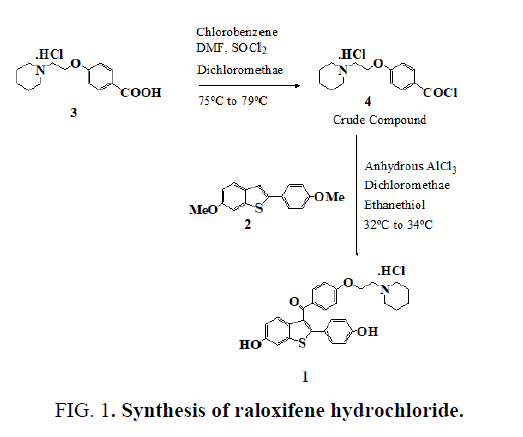
Raloxifene hydrochloride [1], is an estrogen agonist/antagonist, commonly referred to as a Selective Estrogen Receptor Modulator (SERM) [1,2] that belongs to the benzothiophene class of compounds. Raloxifene decreases the resorption of bone and reduces the biochemical markers of bone turnover to the premenopausal range [3-5]. Raloxifene hydrochloride may also lower the chance of developing a certain type of breast cancer (invasive breast cancer) in post-menopausal women [6,7]. It can be synthesized [3] directly from aroylation of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene [2] by the acid chloride(4) of 4-[2-(1-piperidinyl)ethoxy]benzoic acid hydrochloride [3] in the presence of AlCl3 followed by addition of ethanethiol (FIG. 1).
Experimental Section
4-[2-(1-Piperidinyl)ethoxy]benzoic acid hydrochloride [3] and 6-methoxy-2-(4-methoxyphenyl) benzo[b] thiophene [2] were prepared by procedures reported previously [3]. Decanethiol was from commercial source. All melting points are uncorrected and were determined in capillary tubes on an Electothermal melting point apparatus. 1H NMR spectra were recorded on a Brucker ADVANCE 400 MHz spectrometer, using DMSO-d6 as solvent and TMS as internal standard. Electrospray ionization mass spectroscopy was performed using an ion trap mass spectrometer (Model 6310 Agilent). All reactions were monitored and checked by Thin Layer Chromatography (TLC) using methanol and spots examined by a UV lamp.
Preparation of [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl][4-[2-(1-piperidyl)ethoxy]phenyl] methanone hydrochloride (Raloxifene hydrochloride) [1]
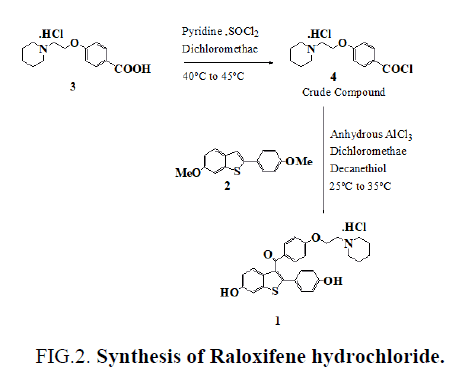
To a solution of 4-[2-(1-piperidinyl)ethoxy]benzoic acid hydrochloride (3) (14.3 g, 0.05 mol) in methylene dichloride (400 mL) and pyridine (0.5 mL) at 25ºC to 35ºC, thionyl chloride (23.8 g, 0.20 mol) was added dropwise under argon for 15-30 minute. The reaction mixture was stirred for 2 hr. at 40ºC to 45ºC. Excess thionyl chloride and solvent were removed in vacuum at 40◦C to afford 15.0 g of the crude acid chloride hydrochloride salt [4]. The crude solid acid chloride hydrochloride [4] was dissolved in methylene dichloride (150 mL), cooled to 0ºC to 10ºC, 6-methoxy-2-(4-methoxyphenyl)benzo[b] thiophene [2] (10.8 g, 0.04 mol) was added. Then, anhydrous aluminium chloride (37.0 g, 0.28 mol) was added portion wise over a period of 30 min and then the mixture was allowed to warm to 30ºC and stirred for 2 hr at 25-35ºC. Then decanethiol (28.0 g, 0.16 mol) was added and stirred for 2 hr. at 25-35ºC. The reaction mixture was quenched with mixture of methanol (100 mL), ice (200 g) and Conc. HCl (15 mL) and stirred for 1 hr. at 25-35ºC. The precipitated solid was collected, washed with water (100 mL X 2) and dried at 65ºC for 4 h to afford 20.0 g of crude compound 1, which was crystallized from methanol/water (23/1, vol/vol) to yield 13.6 g of compound 1 (53.3 %yield) as a white solid, MP 258-260°C, liter 3, 258°C ; 1H NMR: δ 1.34, 1.72 [2H, m, (CH2CH2)2CH2], 1.76 [4H, m, N(CH2CH2)2], 2.96 (2H, m, N-CH2), 3.43 [4H, m, N(CH2CH2)2], 4.44 (2H, m, O-CH2), 6.67 (2H, d, Ar), 6.85 (1H, d, Ar), 6.95 (2H, d, Ar), 7.18 (2H, d, Ar), 7.25 (1H, d, Ar), 7.35 (1H, s, Ar), 7.70 (2H, d, Ar), 9.77 (1H, s, OH), 9.82 (1H, s, OH), 10.16 (1H, brs, NH), MS (ESI): m/z 474.6 (M +H). “This procedure has been scaled up using 250g of compound 1.”
Results and Discussion
Commonly used thiols like ethanethiol and benzyl mercaptan in demethylation reactions have a foul smell making them difficult and unpleasant to use in the laboratory without fume hoods. The problem becomes even worse in industry on a large scale. Odorless substitutes are therefore always required. Few papers [8,9] discuss the use of long chain thiols to minimize odor, so we used this work as a basis for choosing a long chain thiol for our demethylation reaction. We now report a new, highly active demethylation reagent, an aluminum chloride and decanethiol, characterized by rapid action under mild conditions, easy workup of the reaction product, and high yield (FIG. 2.).
Conclusion
In conclusion, we have found that decanethiol is odorless thiol compared to ethanethiol. We believe that removing the foul-smelling thiols and use of these odorless thiols will greatly improve the greenchemistry.
Acknowledgement
The authors are thankful to Tyche Industries Ltd for financial support.
References
- Grese TA, Dodge JA. Selective Estrogen Receptor Modulators (SERMs). Curr Pharm Des. 1998;4:71-92.
- Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
- Jones CD, Jevnikar MG, Pike AJ, et al. Antiestrogens. 2. Structure-activity studies in a series of 3-aroyl-2-arylbenzo [b] thiophene derivatives leading to [6-hydroxy-2-(4-hydroxyphenyl) benzo [b] thien-3-yl]-[4-[2-(1-piperidinyl) ethoxy] phenyl] methanone hydrochloride (LY 156758), a remarkably effective estrogen antagonist with only minimal intrinsic estrogenicity. J Med Chem. 1984;27:1057-66.
- Sato M, Grese TA, Dodge JA, et al. Emerging therapies for the prevention or treatment of postmenopausal osteoporosis. J Med Chem. 1999;42:1-24.
- Draper MW, Flowers DE, Huster WJ, et al. A controlled trial of raloxifene (LY139481) HCl: impact on bone turnover and serum lipid profile in healthy postmenopausal women. J Bone Miner Res. 1996;11:835-42.