Original Article
, Volume: 14( 1)In vitro Antimicrobial Potential of Coturnix japonica Egg Shell and Membrane Powder
- *Correspondence:
- Irokanulo EO , Department of Biological Science, Microbiology Programme, Landmark University, PMB 1001 Omu-Aran, Kwara State, Nigeria, Tel: +2347014720491; E-mail: eoirokanulo@yahoo.co.uk
Received: November 10, 2017; Accepted: December 12, 2017; Published: December 20, 2017
Citation: Irokanulo EO, Nwonuma CO, Chukwudi FC. in vitro Antimicrobial Potential of Coturnix japonica Egg Shell and Membrane Powder. Nat Prod Ind J. 2017;14(1):115
Abstract
The Japanese quail (Coturnix japonica) egg shell and membrane powder, though not fully investigated, have been used by indigent peoples for treatment of dermatitis. We conducted an in vitro study of the antimicrobial potential of the crude Japanese quail egg shell and membrane, ground into powder, against two bacterial isolates; Pseudomonas aeruginosa (ATCC6633) and Staphylococcus aureus (ATCC6538) and four local isolates of fungi; Aspergillus flavus, Aspergillus fumigatus, Microsporum audouinii and Microsporum gypseum. The assays were based on agar diffusion and agar dilution methods for the bacterial and fungal organisms respectively. Photometric technique was used to determine minimum inhibitory concentration (MIC) of the powder. Antimicrobial activity of the powder was observed against the two bacterial isolates and the four fungi when compared to the control drugs; ciprofloxacin and fluconazole used, respectively. Though the egg shell and membrane were used in their crude form, the result obtained demonstrated an antimicrobial potential of the powder. Further assessment of this natural product for use in the treatment of skin infections resulting from bacterial and fungal agents is suggested.
Keywords
Natural product; Antimicrobial; Quail; Egg shell membrane; in vitro
Introduction
Use of natural products for the development of antimicrobials has become attractive because of their apparent low toxic effects on human cells than those synthetically produced. This shift has gained tremendous momentum and applications world-wide.
This new approach to health care management and delivery which is now prominent in both the developed and developing countries is appreciated more in countries where dearth of resources for health care still persists and where a large proportion of the populations originally rely on ethno-medicinal products to meet their primary food and health care needs [1]. In the search for effective antimicrobials, various natural products of plant and animal origin are being tested; an approach that is further fuelled by the emergence of multi-drug resistant varieties of infectious microorganisms.
Extracts from natural organic products found to be efficacious are isolated for use in the production of pharmaceuticals [2-4]. Among the natural products that have been used is egg from avian species. The embryonic sac from chicken egg has found use in other areas due to its rich protein content. It has been used in medicine and even for gardening [5-7].
The yolk contains lysozyme and albumen, with small traces of other proteins in the shell cuticle. These constituents possess potent antimicrobial properties. Other constituents of the egg that have antimicrobial properties include the matrix proteins and polysaccharides, also found in the eggshell cuticle and egg membrane [8-10]. Studies indicate that some of these matrix proteins are able to prevent the passage and growth of Gram negative spoilage bacteria within the egg [11]. Egg membrane protein is also used in cosmetic products [12].
The eggshell is a complex, multifunctional biomineral material with an organic phase of lipids and proteins and is made up of polysaccharides, hydroxyapatite crystals, lipids and glycoprotein with antimicrobial principles [13]. The quail egg and its shell by-product are believed to possess inherent nutritional and medicinal values and have become very popular for numerous health and nutritional benefits; though most of the reports on these are yet to be scientifically ascertained. Despite their small size, the nutritional value of the quail egg is about three to four times that of the chicken egg. They contain a lot more proteins, vitamins, iron and potassium than in chicken and duck eggs [14]. Reports indicate that a little portion of the quail egg shell or its membrane placed on a black head; acne or pimple can erase such skin blemishes and can cure superficial infections [15]. Previous studies have shown that extracts of avian eggshell cuticle evaluated for their antimicrobial activity demonstrated bactericidal activity against both Gram-positive and Gram-negative bacteria [13,16]. Dermatophytes cause infections of the skin, hair and nails due to their ability to obtain nutrients from keratinized material. Examples of common dermatophyte infections include athlete's foot; also known as tinea pedis and jock itch known as tinea cruris [17]. Dermatitis and other related superficial infections are prevalent in rural communities due to poor hygiene. The prevalence of dermatitis is also aided by the occupation of individuals; especially the rural poor where occupations such as farming is very popular often accompanied by a stress induced depression of the immune system that engender the development of skin infections. Dermatitis is often caused by ubiquitous bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa, viruses such as herpes simplex and the dermatophyte fungi [18]. Microsporum, Epidermophyton and Trichophyton are the most common genera of anamorphic fungi implicated in skin infections.
Based on previous though scientifically unsubstantiated reports of the curative property of the quail eggshell, this study seeks to determine the in vitro antimicrobial property of the egg shell powder on some microorganisms often associated with the primary or secondary causes of skin infections.
Materials and Methods
Collection and processing of eggs
Coturnix japonica eggs were obtained from the poultry farm of the National Veterinary Research Institute Vom, Plateau State, Nigeria. The eggs were stored dust free at 4°C until used.
The outside of the egg shells were gently but properly washed with clean water to decontaminate and remove adhering debris and the surface rinsed off using sterile distilled water. The washed eggs were allowed to air-dry and then cracked open and the contents emptied into a clean sterile receptacle.
The egg shells were soaked in sterile clean water for one hour to wash off albumin residue followed by another jet of sterile distilled water to rinse the washed shells. The washed shells were left to air-dry and then placed in glass jars in the oven at 30°C for 24 h to dry to constant weight.
The dry egg shells were crushed in a clean sterile porcelain mortar and further ground in a Warren blender into fine powder. The resultant powder from the shells was stored in disposable sterile glass jars.
Antibacterial activity
The egg shell powder was used in the crude state without extraction. The antibacterial activity of the powder was carried out on nutrient agar incorporated with 0.8% sodium nitrate in Petri dishes. The agar medium was later seeded with 1 ml of 103 CFU/ml Pseudomonas aeruginosa (ATCC6633) and Staphylococcus aureus (ATCC6583) suspensions spread evenly over the surface of the agar and then allowed to dry in the incubator at 37°C. Using the ditch method, wells measuring 0.5 mm in diameter were bored in the inoculated agar medium using a sterile agar punch. Four concentrations of the egg shell powder solution; 1000 mg, 500 mg, 250 mg and 125 mg, were each prepared in 10 ml sterile distilled water and properly mixed using a Vortex-mixer (SA8) at 600 rpm for 60 seconds. The mixtures were covered and left to stand at ambient temperature (28°C) for 48 h before use. The wells in the inoculated agar were thereafter filled with 0.1 ml; equivalent of 10 mg, 5.0 mg, 2.5 mg and 1.25 mg solution of the egg shell powder per well. Similarly, 0.1 ml (10 mg equivalent) solution of the control drug ciprofloxacin (fluoroquinolone), a widely used antibacterial drug was added to the control wells. The test was carried out in replicates of three.
Determination of minimum inhibitory concentration (MIC) for bacterial organisms
Two sets, A and B, of egg shell powder solutions of four different concentrations were prepared by weighing; 100 mg, 50 mg, 25 mg and 12.5 mg of powder and reconstituting in 9 ml of nutrient broth respectively in sterile Universal bottles. To the first set A, was added 1 ml each, of 103 CFU/ml of the bacterial suspension Staphylococcus aureus (ATCC6583) and to the second set of tubes B, 1 ml each of 103 CFU/ml of the bacterial suspension Pseudomonas aeruginosa (ATCC6633) was added. All were mixed thoroughly on a vortex machine resulting to final concentrations of 10 mg/ml, 5 mg/ml, 2.5 mg/ml and 1.25 mg/ml of the egg shell powder. The absorbance of each powder-organism mixture was determined immediately using the spectrophotometer (Jenway UV/Vis spectrophotometer 6705) at a wavelength of 500 nm. Following this, the mixture of egg shell powder and bacteria organisms were incubated at 37°C for 24 h after which the absorbance of the samples were read again at the same wavelength. Both readings were recorded and compared to determine the lowest concentration with the least change in absorbance. Prior to the readings, the broth was used as the blank while the different concentrations of the egg shell solution as stated above, was used as reference for the test.
Antifungal activity
The antifungal activity of the shell powder was tested on four fungal isolates; Aspergillus flavus, Aspergillus fumigatus, Microsporum gypseum, Microsporum audouinii using the agar dilution method as described by Ncube et al. [19].
Four sets of Sabouraud dextrose agar medium were prepared for the test and control. Briefly, to 20 ml freshly prepared Sabouraud dextrose agar (LAB-009) culture medium in Petri dishes at 40°C was added 1 ml each of 200 mg, 150 mg, 100 mg, 50 mg, 25 mg and 12.5 mg of the quail egg shell powder solutions and another with 1 ml of 200 mg of fluconazole in sterile distilled water as control drug. These were well mixed by rotation on the bench and allowed to solidify. The final concentrations of the egg shell powder were 10 mg/ml, 7.5 mg/ml, 5 mg/ml, 2.5 mg/ml, 1.25 mg/ml, 0.625 mg/ml respectively while that of fluconazole was 10 mg/ml. Each agar plate was then inoculated with 0.1 ml of the spore suspensions containing 103/ml of each of the fungi to be tested. The inoculated plates were placed in a 37°C incubator for 6 h for the spores of the fungi to adsorb onto the surface of the culture medium. The plates were thereafter incubated at 25°C for 14 days and examined for fungal growth through mycelia formation beginning 24 h after incubation. Sensitivity was indicated by a no growth of the fungus on the plate. Negative controls were included for the four fungi; separate SDA plates were inoculated with each of the four species of fungi with no quail egg shell powder or fluconazole added. All the tests were in replicates of three.
Determination of minimum inhibitory concentration (MIC) for fungal organisms
The minimum inhibitory concentration of the quail egg shell powder for the four fungi tested was carried out using similar protocol for the bacteria organisms using concentrations of the egg shell powder; 10 mg/ml, 7.5 mg/ml, 5.0 mg/ml, 2.5 mg/ml and 1.25 mg/ml in 10 ml amounts in potato dextrose broth and inoculated with 1 ml (103) of the spore suspensions of each of the fungi and incubated for 14 days. Absorbance readings followed same protocol as in the bacterial MIC determination.
Results
The Quail egg shell powder showed antibacterial and antifungal activities on the two bacterial organisms; Pseudomonas aeruginosa and Staphylococcus aureus and on the four fungi: Aspergillus flavus, Aspergillus fumigatus, Microsporum audouinii and Microsporum gypseum.
Antibacterial activity
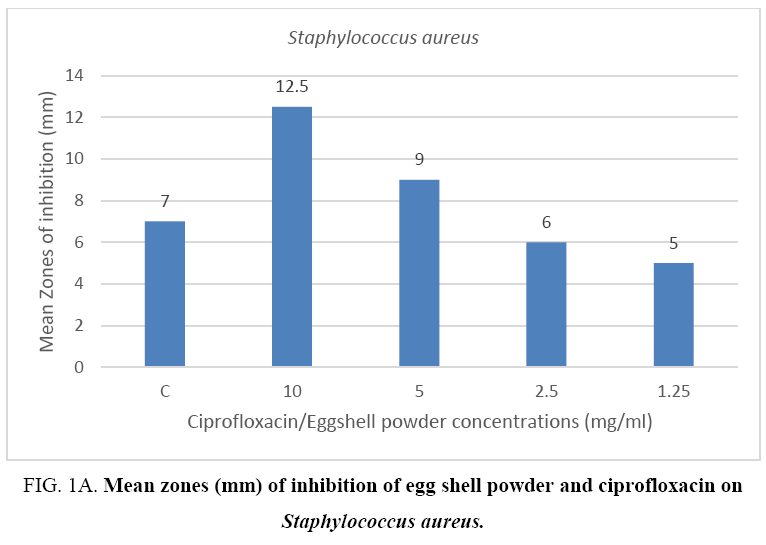
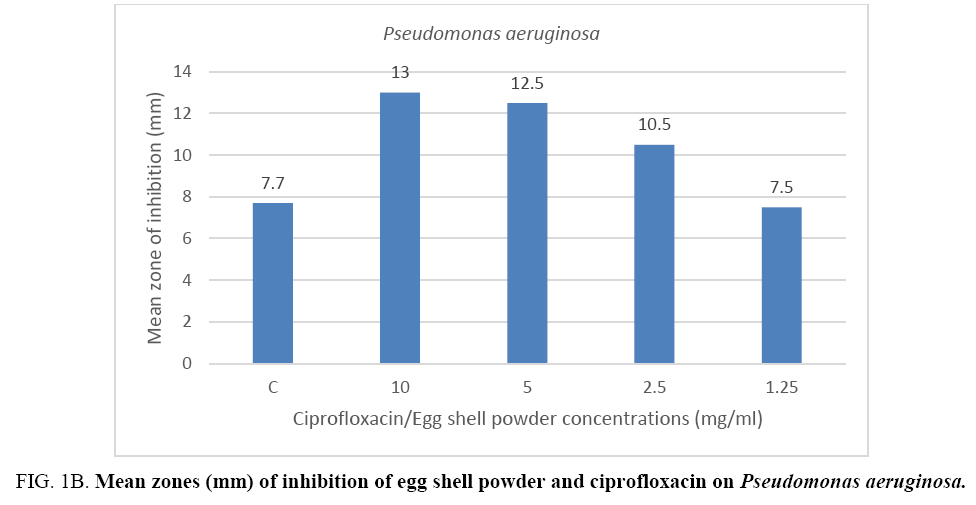
The result from the mean zones of inhibition between the Gram positive bacteria (Staphylococcus aureus) and the Gram negative bacteria (Pseudomonas aeruginosa) showed no appreciable difference when compared per concentration respectively. On the other hand, results of the control drug ciprofloxacin when compared to the mean zones recorded for the 10 mg eggshell powder against the two bacterial species showed a difference in favor of the quail eggshell powder for test organism Pseudomonas aeruginosa (Figure 1A, 1B).
Figure 1A: Mean zones (mm) of inhibition of egg shell powder and ciprofloxacin on Staphylococcus aureus.
Figure 1B: Mean zones (mm) of inhibition of egg shell powder and ciprofloxacin on Pseudomonas aeruginosa.
Antifungal activity
The antifungal activity results recorded as inhibitory had no fungal growth at 21 days in all three replicates after incubation. Higher concentrations of the Quail egg shell powder had more inhibitory effects than lower concentrations on all of the fungal organisms. The control drug (fluconazole-10 mg) inhibited Aspergillus flavus and Microsporum audouinii while Aspergillus fumigatus and Microsporum gypseum were not inhibited by the fluconazole. All the fungal growths recorded occurred beginning at 10 days after incubation.
Minimum inhibitory concentration (MIC) for bacterial isolates
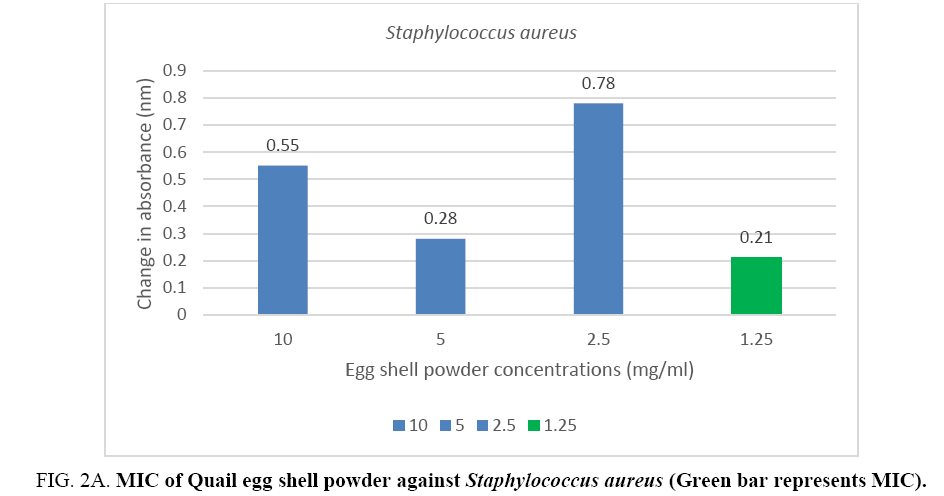
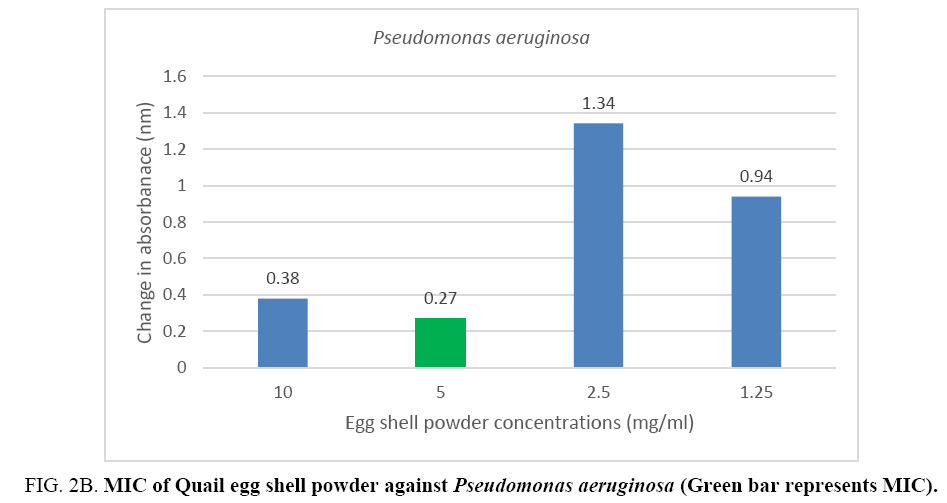
The minimum inhibitory concentration (MIC) of the Quail egg shell powder for bacterial isolates varied according to organism: 12.5 mg (1.25 mg/ml) for Staphylococcus aureus and 50 mg (5 mg/ml) for Pseudomonas aeruginosa (Figure 2A, 2B).
Figure 2A: MIC of Quail egg shell powder against Staphylococcus aureus (Green bar represents MIC).
Figure 2B: MIC of Quail egg shell powder against Pseudomonas aeruginosa (Green bar represents MIC).
Minimum inhibitory concentration (MIC) for fungal isolates
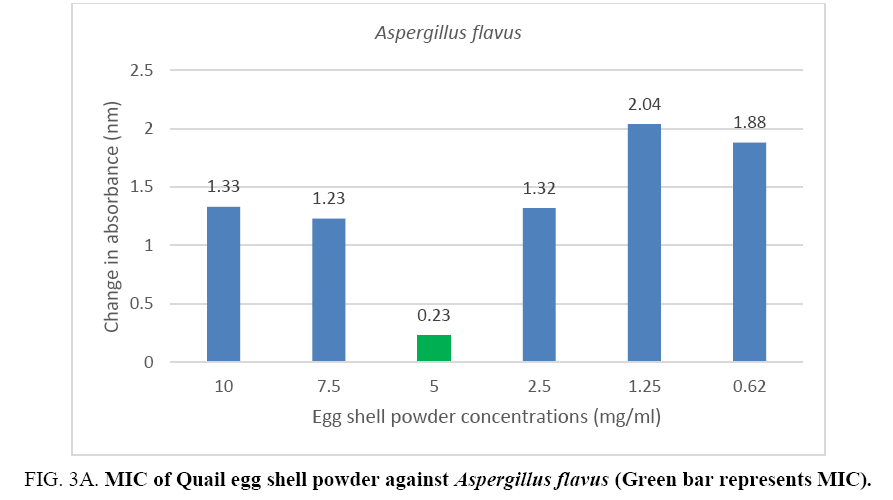
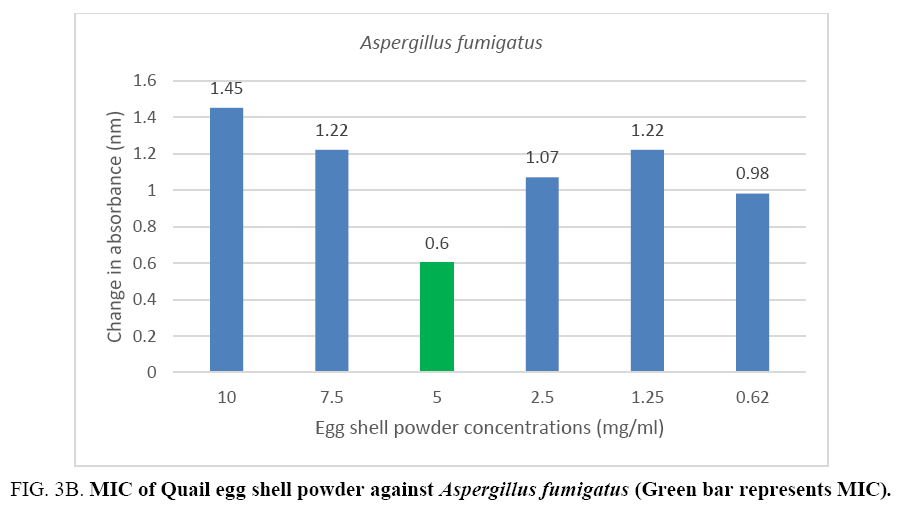
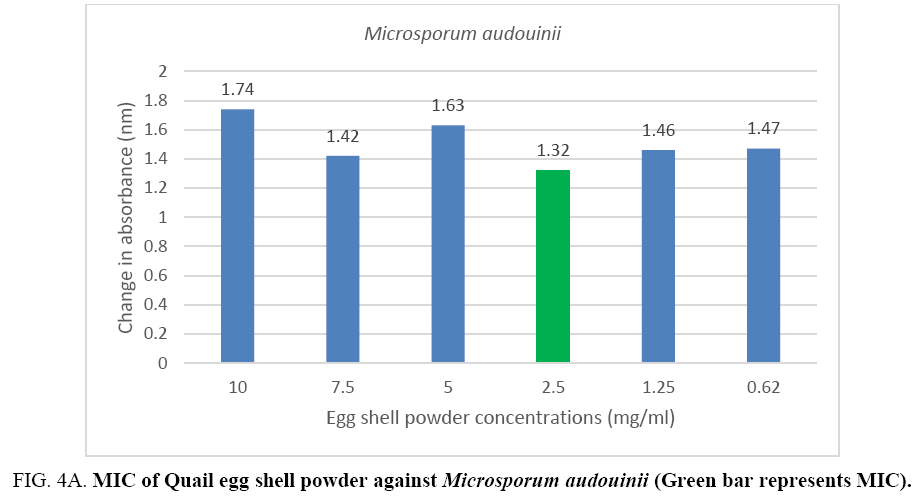
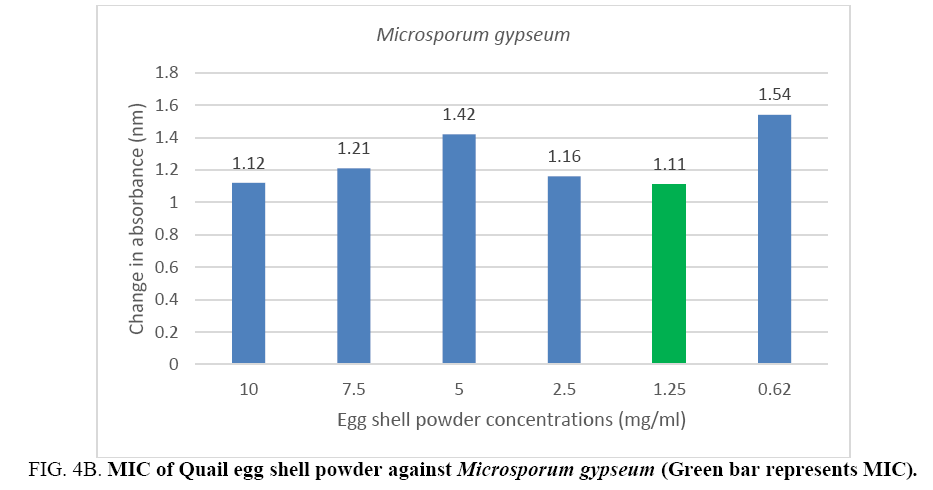
An MIC of 5.0 mg/ml was recorded for Aspergillus flavus and Aspergillus fumigates (Figure 3A, 3B) while Microsporum audouinii and Microsporum gypseum had MICs of 2.5 mg/ml and 1.25 mg/ml respectively (Figure 4A, 4B).
Figure 3A: MIC of Quail egg shell powder against Aspergillus flavus (Green bar represents MIC).
Figure 3B: MIC of Quail egg shell powder against Aspergillus fumigatus (Green bar represents MIC).
Figure 4A: MIC of Quail egg shell powder against Microsporum audouinii (Green bar represents MIC).
Figure 4B: MIC of Quail egg shell powder against Microsporum gypseum (Green bar represents MIC).
Change in Absorbance (nm)=A0; (A2 – A1)
A1=Absorbance before incubation
A2=Absorbance after 48 h incubation
A0=Difference (A2-A1).
Change in Absorbance (nm)=A0; (A2 – A1)
A1=Absorbance before incubation
A2=Absorbance after 48 h incubation
A0=Difference (A2-A1).
Discussion
A host of health conditions are believed to be treatable by quail egg. Most of these claims are yet to be proved through Scientific research methods. For instance, eggs, particularly from the Japanese quail are reported to help in the treatment of tuberculosis, bronchial asthma and diabetes. According to Ronjit et al., [1] the Indians prepare ash from leftovers of hatched chicken egg shells which they use in the treatment of persons perceived to be suffering from food poisoning.
Based on our findings in this in vitro study, we report that the powder from egg shell and membrane from the Japanese quail (Coturnix japonica) has antibacterial and antifungal properties. We note also that both bacterial organisms tested; Gram positive (Staphylococcus aureus) and Gram negative (Pseudomonas aeruginosa), were inhibited by the crude powder solution of the egg shell. This finding lends credence to earlier reports of medicinal use of the quail egg shell [15]. Egg shell contains significant amount of bio-available calcium. Apart from its bone enhancing and building properties, calcium neutralizes acid. This acid neutralizing property of calcium play a role in altering the acidic growth environment required by fungi to alkaline which is inhibitory to their survival. Eggshells therefore, it has been said, may just be as important as the eggs inside them that we so very much cherish. Our report further support claims of use of the egg shell powder in its crude form for local treatment of dermatitis.
In this study, the membrane attached to the egg shell, known for protecting the contents of the egg from bacterial invasion and which also prevents rapid evaporation of liquid from the egg was ground together with the shell [20,21]. This egg membrane contains ovotransferrin which is a glycoprotein found in the egg albumen; the lipophilic principles are added into the egg during the egg’s development [22]. This protein is heat tolerable and is often referred to as “heat shock protein” and plays a key role as an antimicrobial agent in protecting developing embryos from bacterial infection.
Conclusion
The inhibitory activity of the quail egg shell powder on the two bacterial and four fungi tested as observed in this study therefore, can be attributable in part, to the antimicrobial properties of the ovotransferrin: which has an iron-binding property that aids the membrane of the egg shell to deprive bacteria of free iron for their metabolism and replication; thus inhibiting its growth and also to the high calcium content of the shell which provides a veritable alkaline environment that is inimical to fungal growth. As noted earlier, the high alkaline content of the egg shell due to calcium negates the acid environment that promotes fungal growth by neutralizing the acid in the environment. We intend to further evaluate extracts of the egg shell using different solvents to help elucidate the principles and active peptides involved for other uses. Following our findings from this study, we recommend the use of the eggshell powder, either alone or as part of a formulation as an antimicrobial preparation for topical application against opportunistic organisms in skin infections.
References
- Ronjit R, Robindra T, Tamuli KA, et al. Traditional zoo therapy practiced among the Karbis of Assam (India). Department of Life Science, Assam University, Diphu Campus, Diphu, KarbiAnglong, Assam Department of Zoology, Cotton College, Guwahati-781 001, Assam. The Ecosan: Special Issue. 2011;1:161-66.
- Azhari EE, Abdmageed MAM, Shyoub ME, et al. Evaluation of toxicity of some plants having traditional uses in Sudan on brine shrimp. Global J Trad Med Sys. 2013;2(1):19-23.
- Farnsworth NR, Soejarto DD. Global importance of medicinal plants. The Conservation of Medicinal Plants. 1991; 26:25-51.
- Arora S, Kaur K, Kaur S. Indian medicinal plants as a reservoir of protective phytochemicals. Teratogenesis, Carcinogenesis and Mutagenesis. 2003;23(S1):295-300.
- Agriculture and Agri-food Canada (AAFC). Little known uses for eggs. Sub-sector reports.Canada.ca 2013.
- Pleasant B. Keeping Chickens as Part of a Healthy Vegetable Garden. 15 November 2012 [cited 2017 Aug 12]. Available from: https://www.growveg.co.uk/guides/keeping-chickens-as-part-of-a-healthy-vegetable-garden/
- OVM and the Miracle of Eggshell Membrane. [cited 2017 Aug 22]. Available from: https://foreveryoung.perriconemd.com/ovm-and-the-miracle-of-eggshell-membrane.html
- Nakano T, Ikawa N, Ozimek L. Extraction of glycosaminoglycans from chicken eggshell. Poultry Science. 2001;80(5):681-4.
- Gautron J, Hincke MT, Panheleux M, et al. Ovotransferrin is a matrix protein of the hen eggshell membranes and basal calcified layer. Connective Tissue Research. 2001;42(4):255-67.
- Nakano T, Ikawa NI, Ozimek L. Chemical composition of eggshell and shell membranes. Poult Sci. 2003;82:510-14.
- Valenti P, Antonini G, Von Hunolstein C, et al. Studies of the antimicrobial activity of ovotransferrin. International Journal of Tissue Reactions. 1983;5(1):97-105.
- Froning GW. Recent Advances in Egg Products Research and Development. University of California Egg Processing Workshop; 1998 June 2-3; Riverside and Modesto.
- Wellman-Labadie O, Picman J, Hincke MT. Antimicrobial activity of the Anseriform outer eggshell and cuticle. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2008;149(4):640-9.
- Tunsaringkarn T, Tungjaroenchai W, Siriwong W. Nutrient benefits of quail (Coturnix japonica) eggs. International Journal of Scientific and Research Publications. 2013 May;3(5):1-8.
- Truffier JC. Approach to treatment of allergy by consumption of quail eggs. La Clinique [Internet]. 1978Mar [cited 2017 Aug 18]. Available from: www.ovogenics.eu/es/page/jc-truffier-treatment-of-allergy.html.
- Wellman-Labadie O, Lemaire S, Mann K, et al. Antimicrobial activity of lipophilic avian eggshell surface extracts. Journal of Agricultural and Food Chemistry. 2010;58(18):10156-61.
- Centers for Disease Control and Prevention [Internet]. Ringworm; December 4, 2015 [cited 2017 Aug 27]. GA: U.S. Department of Health & Human Services. Available from: https://www.cdc.gov/fungal/diseases/ringworm/index.html.
- National Eczema Association. [Internet]. Eczema Facts; [cited 2017 Aug 27]. CA: National Eczema Association. Available from: https://nationaleczema.org/research/eczema-facts/.
- Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African Journal of Biotechnology. 2008;7(12).
- Novak CL, Troche C, Jamison K. Poultry: Beginning of Life.
- King’Ori AM. A review of the uses of poultry eggshells and shell membranes. Int J Poult Sci. 2011;10(11):908-12.
- Wellman-Labadie O, Picman J, Hincke MT. Antimicrobial activity of cuticle and outer eggshell protein extracts from three species of domestic birds. British Poultry Science. 2008;49(2):133-43.