Research
, Volume: 15( 1) DOI: 10.37532/0974-7435.2019.15(1).185Identification and Characterization of an Indigenous Hydrocarbonoclastic Bacterial Strain Stenotrophomonas maltophilia newly Isolated from Seawater and Marine Sediments of Oran Harbor Northwestern Algeria
- *Correspondence:
- Faiza Bendadeche Laboratory of Aquaculture and Bioremediation (AquaBior), Department of Biotechnology, Faculty of Nature and Life Sciences, University of Oran 1 Ahmed Ben Bella, Oran, Algeria E-Mail: faizabendadeche@yahoo.com
Received: May 18, 2018; Accepted: January 19, 2019; Published: January 30, 2019
Citation: Bendadeche F, Baba Hamed MB, Abi Ayad SMEA. Identification and Characterization of an Indigenous Hydrocarbonoclastic Bacterial Strain Stenotrophomonas Maltophilia Newly Isolated from Seawater and Marine Sediments of Oran Harbor, Northwestern Algeria. Biotechnol Ind J. 2019;15(1):185.
Abstract
Microorganisms have an important role in the bioremediation of petroleum contaminants that cause great concern about their persistent toxicity, carcinogenicity and difficult elimination. In this study, crude oil-degrading bacterial strain SP54N was isolated from the contaminated marine sediments and seawater at the harbor of Oran, Northwestern Algeria, using Bushnell-Hass salt medium. This strain could support high concentrations of crude oil (up to 10% v/v), and was identified as Stenotrophomonas maltophilia by sequencing and analyzing the 16s rDNA with the BLAST program on the NCBI website. The effects of pH, temperature and salinity on rate growth of strain SP54N, in BHSM medium supplemented with 2% (v/v) of crude oil as sole carbon and energy source, were studied. The results show that maximum growth rate was obtained at pH 7, temperature 25°C and 3% (w/v) of salinity, at 140 rpm. Moreover, Stenotrophomonas maltophilia could effectively utilize crude oil as its sole carbon and energy source. Therefore, Stenotrophomonas maltophilia SP54N can be used as an excellent degrader to develop one eco-friendly and cost-effective method for the bioremediation of harbor of Oran, as indigenous bacteria, and marine environments polluted by oil and petroleum hydrocarbons, and could be useful for biotechnological applications.
Keywords
Bioremediation; Crude oil; 16s rDNA; Marine sediments
Abbreviations
PAHs: Polycyclic Aromatic Hydrocarbons; BHSM: Bushnell Hass Mineral Salt Medium; TSI: Triple-Sugar-Iron
Introduction
Aromatic hydrocarbons and oil are the most important environmental pollutants. Hydrocarbon contamination in seawater and sediments has been considered as an international threat for environmental reasons [1]. Hydrocarbon pollution in the harbor and marine environments emerged from the emission of waste product processing, the utilization production, drilling, transportation and storage of petroleum product and from the oil spill accidents. The discharge of petroleum products in the environment causes environmental damage affecting directly or indirectly ecological systems and human health [2,3]. Several restoration techniques have been tested to eliminate or reduce hydrocarbon contaminants in recent decades. Bioremediation is an important strategy for utilizing biological activities for the rapid removal of environmental pollutants [4]. It has been confirmed that the application to be more appropriate and practical for cleaning-up petroleum hydrocarbons in contaminated harbors and marine environments [1].
Biodegradation by natural microbial population is the best reliable mechanism to eliminate xenobiotic pollutants, like crude oil from the environment [5]. Hydrocarbon-degrading microorganisms living in marine environments would be more adapted for restoring the hydrocarbon contamination in the sea. Various hydrocarbons degrading bacteria were isolated from marine environments such as Psychrobacter sp., Rodoccocus sp., Arthrobacter sp., Microccocus, and Spingomonas. Though, the number of the bacterial species capable of degrading petroleum hydrocarbons isolated from the sea is less than that isolated from sediments [6-8].
Polycyclic Aromatic Hydrocarbons (PAHs) is a group of major environmental xenobiotic pollutants which raise significant concern over there toxicity, teratogenicity and carcinogenicity [9,10]. Previous studies confirmed that enormous bacteria, archaea, and fungi have the petroleum-degrading abilities [11,12]. However, these microorganisms were mainly isolated from terrestrial soils and sediments as well as marine sediments [13].
Our aim in present work is to isolate indigenous hydrocarbonoclastic bacterial strains with the abilities to degrade crude oil from contaminated marine sediments and seawater of Oran harbor and characterize the strain SP54N with its high growth rate on crude oil.
Materials and Methods
Samples collection
Samples of marine sediments and seawater of few millimeters of the surface of the sediments (-40 m depth) were collected, in sterile flasks, at the harbor of Oran, Northwestern Algeria (35°42’ 44’’N; 0°38’28’’W) (FIG. 1), in October, 2013, and transported immediately to the laboratory.
Enrichment, isolation, and selection of hydrocarbon-degrading bacteria
A synthetic Bushnell Hass Mineral Salt Medium (BHSM) was used for isolation of hydrocarbon-degrading bacteria [14]. The pH was adjusted to 7.2 and then sterilized by autoclaving at 121°C for 20 min. After the BHSM was cooled, it was supplemented with 2% (v/v) of crude oil (Supplied by the Hassi-Messaoud oil Refinery, Algeria) as sole carbon and energy source. The crude oil was sterilized by 0.22 μm filter membrane.
From mixed sediments and seawater (of -40 m depth) 2 ml was taken after decantation, and added to 500 ml Erlenmeyer flask containing 100 ml BHSM, and incubated for 7 days at 25°C at 140 revolutions per minute (rpm) in a shaker incubator (Heidolph Inkubator 1000 coupled with Heidolph Unimax 1010, Germany). From the enrichment culture product, 1 ml aliquots were transferred to fresh BHSM medium for subsequent subculture. Then, 1 ml inocula from the flask were streaked on a series of four further subcultures on BHSM medium supplemented with 4%, 6%, 8% then 10% of crude oil (v/v) successively as sole carbon and energy source. Each subculture was incubated at 30°C and 140 rpm for 72 hours. Inoculums from the flask of 10% (v/v) of crude oil were streaked out into BHSM agar medium supplemented with 10% of crude oil (v/v) and incubated at 25°C for 3 to 7 days. Phenotypically different colonies were purified in nutrient agar medium (Sigma-Aldrich, Germany). Pure cultures were then inoculated another time in fresh BHSM medium with 10% (v/v) to be sure that it used crude oil as sole carbon and energy source, then stored at -20°C until characterization.
Morphological and biochemical characteristics
The typical biochemical and physiological characteristics of strain SP54N, including Gram staining, motility (Mannitol mobility medium), respiratory type (meat-liver agar), Triple-Sugar-Iron test (TSI test), Citrate utilization test (Simmons’ Citrate agar), were systematically analyzed according to Bergey’s Manual of determination for bacteriology [15]. All mediums were purchased from Sigma-Aldrich (Germany). The oxidase activity was determined by kit oxidase test (Sigma-Aldrich, Germany) and the catalase activity was determined by bubble formation in a 3% (w/v) hydrogen peroxide solution. All biochemical tests below were performed in triplicate.
Molecular identification
DNA extraction: Genomic DNA of strain SP54N was extracted from grown cells using an EasyPure® Bacteria Genomic DNA Kit (TransBionovo Co., Ltd., China) by following the manufacturer’s instructions. The DNA samples were stored at -20°C until use.
16s rDNA gene sequence analysis: The DNA sample were used as the template for PCR amplification of the 16s rDNA gene using the universal primers ben27F (5’-AGAGTTTGATCCTGGCTC-3’) and ben1492R (5’-GGTTACCTTGTTACGCTT-3’), synthesized by Sigma (Germany). The 16s rDNA gene was amplified with the following mixture: 2 μL of DNA template (20 ng), 1μL of ben27F (25 μM), 1 μL of ben1492R (25 μM), 1.0 μL of dNTP (5 mM), 4.0 μL of MgCL2 (25 mM), 5 μL of 10X buffer solution (20 mM), 0.5 μL of Taq DNA polymerase (5 μL-1), and ddH2O up to final volume of 50 μL. PCR conditions were described as below: 95°C for 5 minutes; 35 cycles, 94°C for 1 minute. PCR products were checked by running 1.5% agarose gel electrophoresis. This 16s rDNA was partially sequenced by Genewiz. The sequence similarity was searched by the National Centre for Biotechnology Information BLAST. Analysis of 16s rDNA gene sequence data was performed using Clustal W and the software package MEGA (Molecular Evolutionary Genetics Analysis) version 7.0 [16,17] and the phylogenetic tree was inferred using neighbor-joining methods.
Effects different culture conditions on the growth of the hydrocarbon degrading isolate
The effects of pH, temperature and medium salinity on the growth rate of the strain SP54N were investigated using BHSM medium with 2% (v/v) of crude oil at 140 rpm shaking rate, in 250 ml sterile flasks with 50 ml BHSM medium.
In the effects of culture conditions tests, initial pH in the medium was adjusted to 6.0, 7.0, 8.0 and 9.0 with 1M HCl or 1M NaOH at 25°C. The incubation temperature was controlled at 20°C, 25°C, 30°C, 37°C, and 40°C at pH 7. The concentration of NaCl in the medium was adjusted to 0%, 3%, 6%, 9%, 12% and 15% (w/v) at 25°C and pH 7. The growth was assessed indirectly by measuring the turbidity (OD 600 nm) with the spectrophotometer (Perkin Elmer Lambda 35 UV/Vis Spectrometer, USA) after 48 hours of incubation.
In the effects of culture conditions tests, initial pH in the medium was adjusted to 6.0, 7.0, 8.0 and 9.0 with 1M HCl or 1M NaOH at 25°C. The incubation temperature was controlled at 20°C, 25°C, 30°C, 37°C, and 40°C at pH 7. The concentration of NaCl in the medium was adjusted to 0%, 3%, 6%, 9%, 12% and 15% (w/v) at 25°C and pH 7. The growth was assessed indirectly by measuring the turbidity (OD 600 nm) with the spectrophotometer (Perkin Elmer Lambda 35 UV/Vis Spectrometer, USA) after 48 hours of incubation.
The strain SP54N was grown at an optimal culture conditions on BHSM supplemented by 3% (w/v) of NaCl and 2% of crude oil (v/v) as sole carbon and energy source at 25°C, pH 7 and 140 rpm on a orbital shaker (Heidolph Inkubator 1000 coupled with Heidolph Unimax 1010, Germany) for about 20 days. Cultures were performed in triplicate in 250 ml Erlenmeyer flasks containing 50 ml of medium, while the growth was assessed by measuring the optical density at 600 nm.
Nucleotide sequence submission
The 16s rDNA gene sequence of the crude oil-degrading bacteria strain SP54N was submitted in the NCBI-Gene Bank under the accession number MG738221.
Results
Isolation of hydrocarbon-degrading bacteria
24 crude oil degrading bacterial strains were isolated from sediments and seawater of Oran harbor, by enrichment culture and dilution subcultures with an increasing concentration of crude oil (2% to 10%, v/v) and purified on a plate. The degradation experiments demonstrated that these isolates exhibited their variable growth rate and degradability of crude oil in the BHSM medium with 2% to 10% (v/v) of crude oil as their sole carbon and energy source. Among, strain SP54N possessed a high growth rate on crude oil as a sole carbon and energy source, and supported a high concentration of crude oil (up to 10% v/v).
Identification of stain SP54N
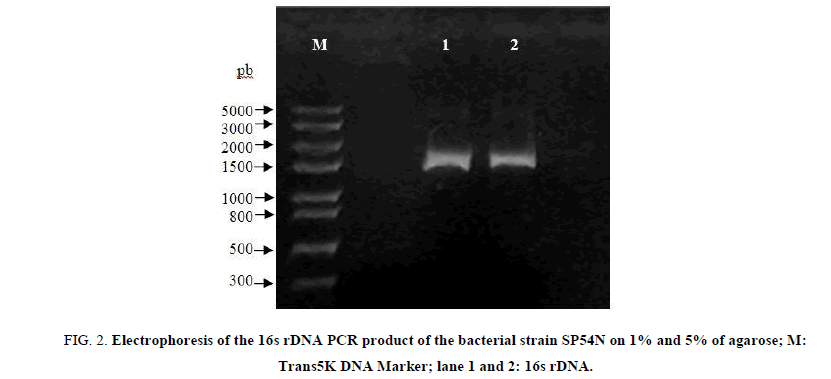
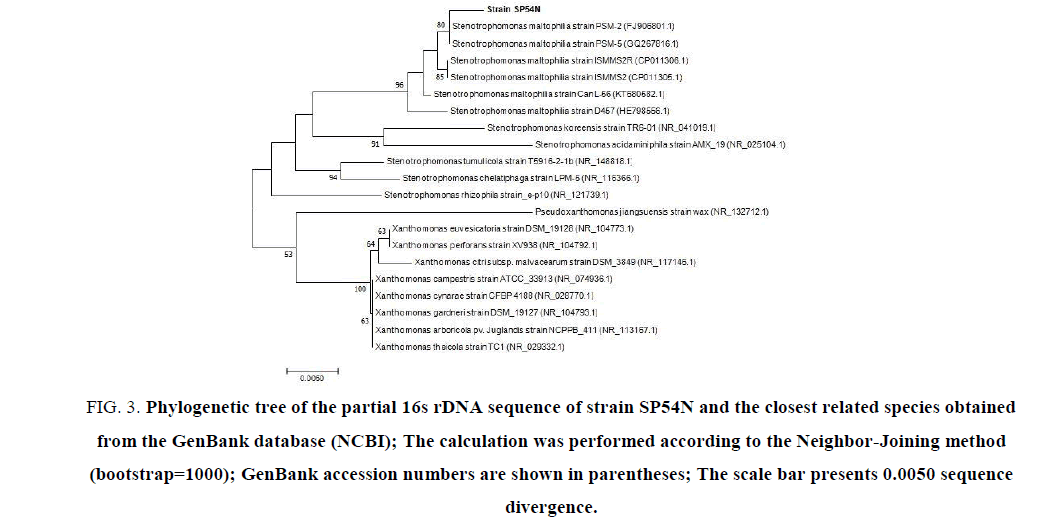
To determine phylogenetic relationships between strain SP54N and other representatives strains of the genus Stenotrophomonas, about1500 pb fragments of the 16s rDNA gene were amplified by PCR (FIG. 2) using universal primers and only 932 pb were sequenced (GenBank ID: MG738221). This continuous stretch sequence was analyzed by the BLAST tool on the National Center for Biotechnology Information (NCBI) website using the Gen Bank database. The calculation of the sequence similarity showed that partial 16s rDNA gene sequence of strain SP54N has a high similarity score of 99% to those of the bacterial strains Stenotrophomonas maltophilia. Subsequently, a phylogenetic tree was constructed using similar 16s rDNA sequences (FIG. 3). The phylogenetic tree was performed using the Neighbor-Joining method [18]. The bootstrap consensus tree inferred from 1000 replicates [19]. The analysis involved 8 nucleotide sequences, codon positions included were 1st+2nd+3rd and all positions containing gaps and missing data were eliminated.
FIG 2: Electrophoresis of the 16s rDNA PCR product of the bacterial strain SP54N on 1% and 5% of agarose; M: Trans5K DNA Marker; lane 1 and 2: 16s rDNA.
FIG 3: Phylogenetic tree of the partial 16s rDNA sequence of strain SP54N and the closest related species obtained from the GenBank database (NCBI); The calculation was performed according to the Neighbor-Joining method (bootstrap=1000); GenBank accession numbers are shown in parentheses; The scale bar presents 0.0050 sequence divergence.
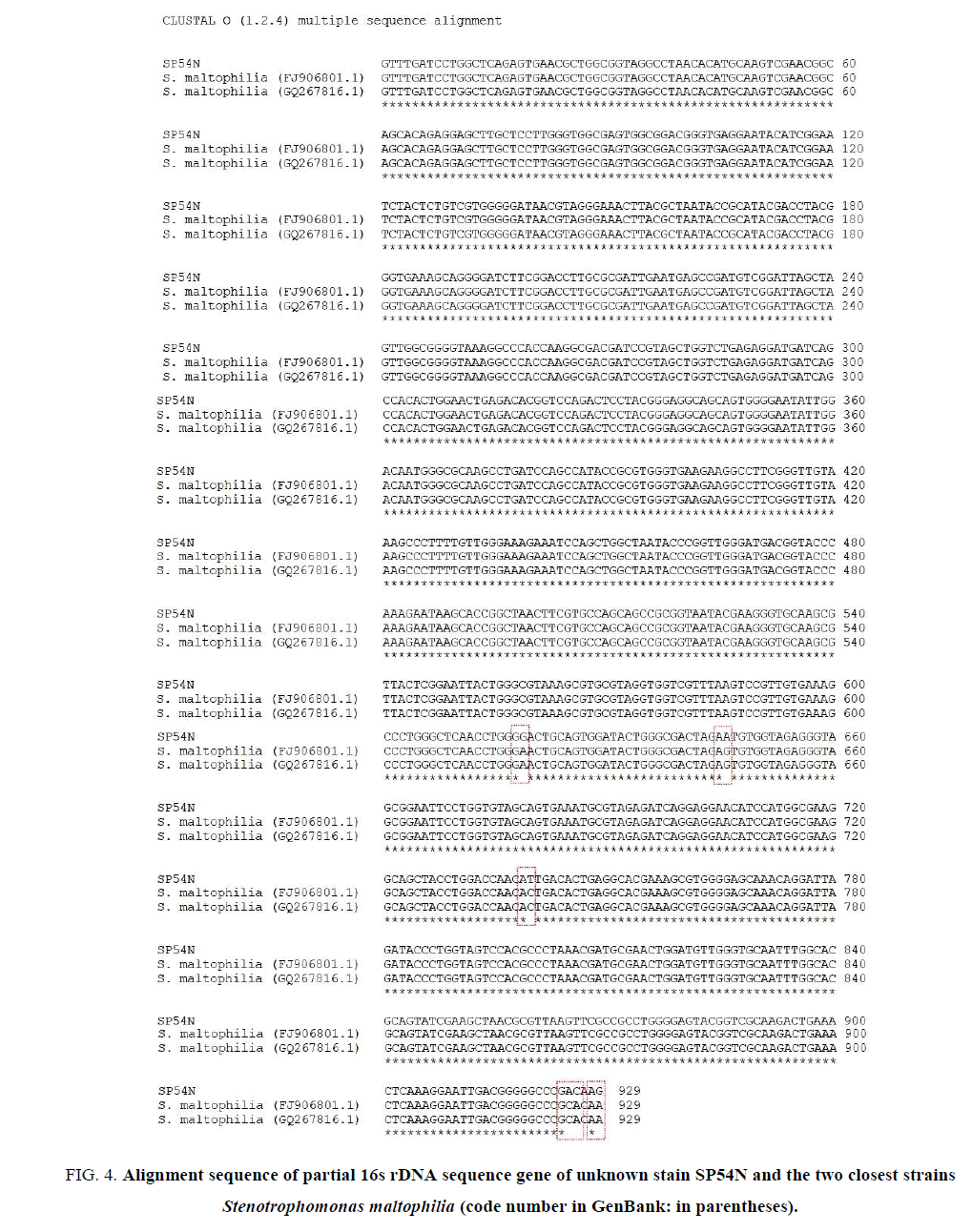
The phylogenetic tree indicated that the bacterial strain SP54N was related species Stenotrophomonas maltophilia (FIG. 4). Thus, strain SP54N was identified as Stenotrophomonas maltophilia. To highlight sequence similarity and differences between strain SP54N and the two closest strains PSM-5 (GQ267816.1) and PSM-2 (FJ906801.1) from GenBank matches, ClutalW multiple sequence alignment was used [15], and results are shown in FIG. 4. Results exposed in FIG. 3 and 4 showed that strain SP54N has some differences with related strains so that it could be a new species of Stenotrophomonas maltophilia.
FIG 4: Alignment sequence of partial 16s rDNA sequence gene of unknown stain SP54N and the two closest strains Stenotrophomonas maltophilia (code number in GenBank: in parentheses).
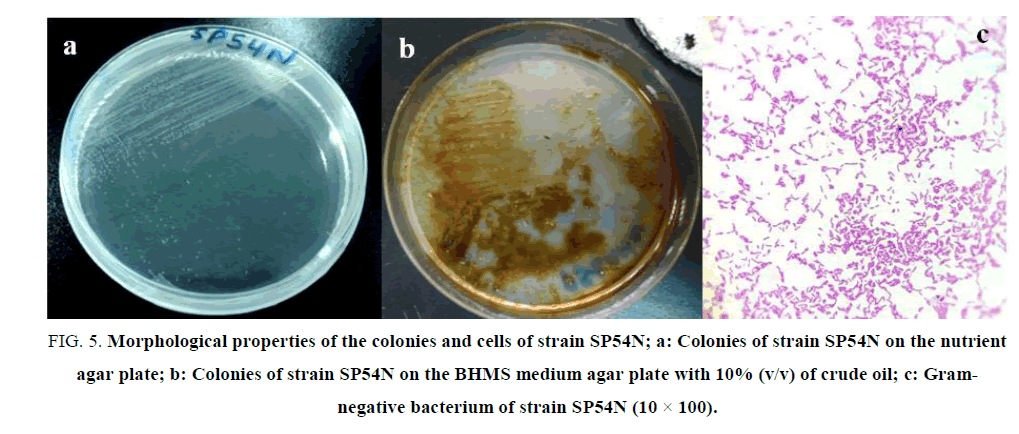
The colonies of strain SP54N were light yellow, circular, smooth, convex, with entire margins and 1 mm to 2 mm in diameter after incubation at 25°C for 24 hours on a nutrient agar plate (FIG. 5a). The gram strain showed that strain SP54N belonged one of the Gram-negative rod-shaped bacteria (FIG. 5c). The FIG. 5b shows colonies of strain SP54N in BHSM agar medium with 10% of crude oil (v/v). The results of the biochemical characteristics of strain SP54N are summarized in TABLE 1.
FIG 5: Morphological properties of the colonies and cells of strain SP54N; a: Colonies of strain SP54N on the nutrient agar plate; b: Colonies of strain SP54N on the BHMS medium agar plate with 10% (v/v) of crude oil; c: Gram-negative bacterium of strain SP54N (10 × 100).
| Characteristics | Results |
|---|---|
| Gram staining | - |
| Shape | Rod-shaped |
| Oxidase test | - |
| Catalase test | + |
| Motility | + |
| Mannitol | - |
| Respiratory type | Strict aerobia |
| TSI test | K/K |
| H2S production | - |
| Gas production | - |
| Citrate utilization | - |
+: Positive reaction; -: Negative reaction
TABLE 1. Morphological and biochemical characteristics of strain SP54N.
Effects of different biodegradation conditions
In order to understand the crude oil degradation environmental characteristics by the isolate strain SP54N, the environmental factors affecting degradation were investigated, including initial pH, temperature, and NaCl concentration.
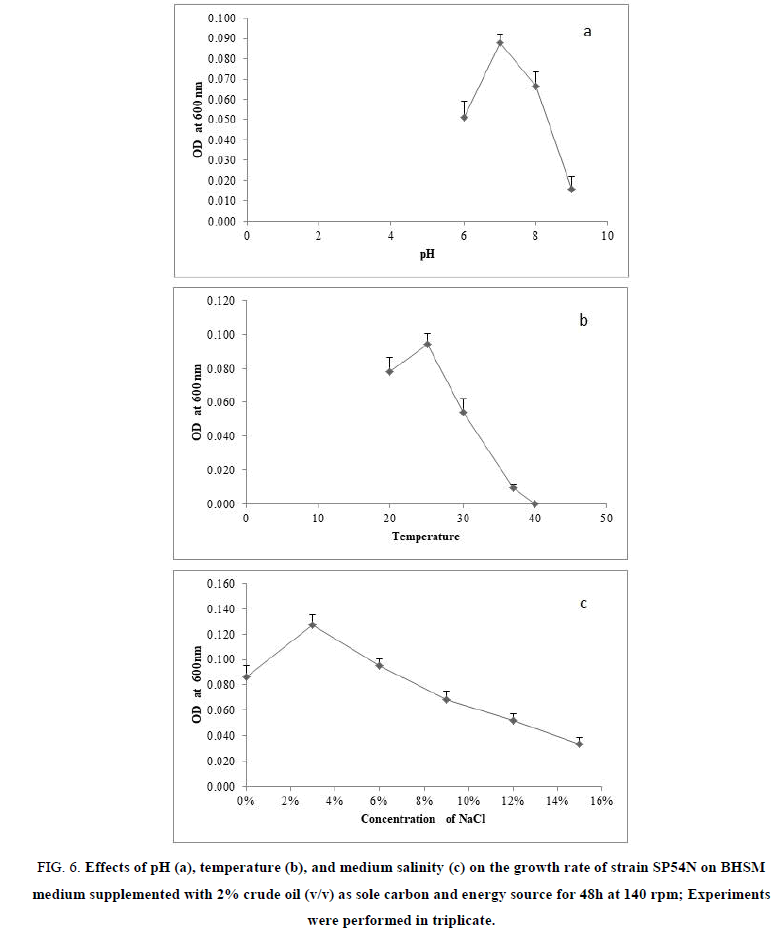
Results obtained from the effect of different pH on the growth rate of strain SP54N in crude oil as a sole carbon and energy source are represented in FIG. 6a. As shown in this curve, the strain could grow at pH 6-9 with the optimum growth rate at pH 7.
FIG 6: Effects of pH (a), temperature (b), and medium salinity (c) on the growth rate of strain SP54N on BHSM medium supplemented with 2% crude oil (v/v) as sole carbon and energy source for 48h at 140 rpm; Experiments were performed in triplicate.
Effects of different temperature in the growth rate of strain SP54N on crude oil as a sole carbon and energy source are illustrated in FIG. 6b. The results demonstrated that this strain could utilize crude oil at temperature 20°C to 37°C, with a maximum growth rate at temperature 25°C. Strain SP54N can grow in temperatures less than 20°C.
Results obtained from the effect of different concentration of NaCl on rate growth of strain SP54N on crude oil as sole carbon and energy source are represented in FIG. 6c. From the curve, it can be seen that the optimum concentration of NaCl for the highest growth rate of strain SP54N was 3% (w/v). However, this bacterial strain can tolerate high salt concentrations (up to 15% w/v).
Growth kinetics of the hydrocarbon degrading isolate
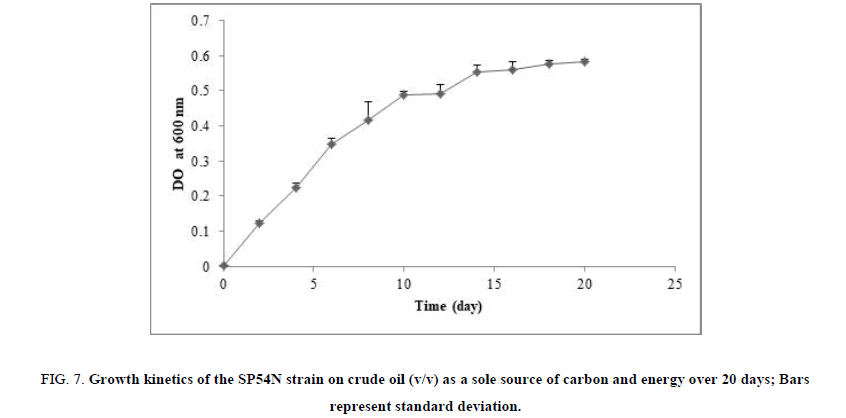
The bacterial stain SP54N was cultured under optimal culture conditions obtained in this study, pH 7, 3% (w/v) of NaCl at 25°C, on BHSM medium with 2% (v/v) of crude oil as sole carbon and energy source over 20 days. The optical density at 600 nm was measured to assess bacterial growth, and the results are shown in FIG. 7. The strain started the logarithmic growth phase from the 1st day to 13th day, and then the stationary phase was attained at day 14.
FIG 7: Growth kinetics of the SP54N strain on crude oil (v/v) as a sole source of carbon and energy over 20 days; Bars represent standard deviation.
Discussion
Strain SP54N was one of the hydrocarbon-degrading bacteria belonging to Stenotrophomonas maltophilia, which was firstly isolated from seawater and sediment of the harbor of Oran-Algeria, and had potential to develop a method to remediate marine environments polluted by petroleum hydrocarbons.
The molecular identification of the SP54N strain was considered. Therefore, the partial sequence of 16s rDNA gene (932 bp) confirmed the identification as Stenotrophomonas maltophilia species with 99% of similarity (FIG. 3 and FIG. 4). Furthermore, the morphological characteristics of strain SP54N were very similar to those of the reported strains Stenotrophomonas maltophilia. The result of the biochemical characteristics of strain SP54N is summarized in TABLE 1.
The study of the effect of culture conditions on petroleum hydrocarbons degradation is very important because it can provide information about the microorganism and their growth requirements, before any contribution for bioremediation. To reveal the crude-oil-degrading conditions of strain SP54N, pH from 6 to 9, temperature from 20°C to 40°C and NaCl concentration from 0% to 15% (w/v) were investigated, as shown in FIG. 6 respectively.
Maximum growth was obtained at pH 7.0 (FIG. 6a). This result is in agreement with those obtained by Neethu et al., [20], Deng et al., [1] and by Kumari et al., [21], who showed that the maximum growth rate of Stenotrophomonas maltophilia, isolated from contaminated soil refinery was at pH 7.5. According to Vandecasteele, extreme pH inhibited the biodegradation of the oil [22]. However, in the marine environment as fresh water, the favorable range of pH was between 7 and 8.
From the curve of FIG. 6b, we note that our isolate strain grows in a wide range of temperature, from 20°C to 37°C (and less than 20°C). These results are according to those obtained with Rouf et al., [23]. Also, Rodriguez-Blanco and Antoine reported that the degradation of crude oil might as well be done at 4°C, 10°C and 25°C with controlled conditions in Mediterranean water [24]. Temperature is the most important factor influencing the biodegradation rate by affecting directly bacterial metabolism [25].
Concerning salinity, it may have a positive impact on hydrocarbon degrading aptitude. There is a correlation between the mineralization rate of several hydrocarbons and salinity [26]. However, contamination by both salt and oil posed a problem for cleaning up using bioremediation, because externally added bacteria do not support high salinity [27].
In this study, results obtained for optimum salinity test showed that strain SP54N supported a high concentration of NaCl and grown in a large range of salinity (0% to 15%, w/v), with a maximum growth rate at 3% (w/v) of NaCl (FIG. 6c). These results are consistent with those obtained by Roder et al., who showed that Stenotrophomonas maltophilia decreased growth rate when exposed to more than 3% (w/v) of NaCl [28].
For results obtained from the kinetics of growth on crude oil by the isolated bacterial strain (FIG. 7), under the optimal conditions, the stationary phase was attained at day 14. These results indicate that this bacterial strain had effectively the ability to degrade crude oil.
Several strains of Stenotrophomonas sp. were previously reported to own their hydrocarbon-degrading capacity [29,30]. Other Stenotrophomonas sp. strains have demonstrated the ability to degrade organic pollutants, such as pesticides, insecticides, chitin, steroid hormones, and Keratin [31-35]. Also, this species possessed the ability to remediate the hydrocarbon contaminated environments [36,37].
Strains of Stenotrophomonas maltophilia were capable of utilizing crude oil as a sole carbon and energy source [38], and could polycyclic aromatic hydrocarbons (HAPs), such as naphthalene, fluorene, and anthracene [39], Phenanthrene [39,40], benzo[a]pyrene [41]. In addition, Urszula, et al., showed that Stenotrophomonas maltophilia (strain KB2) was capable to degrade high concentrations of aromatic compounds, and might utilize phenol as a sole carbon and energy source of, and different kind of aromatic compounds such as protocatechuic acid (3,4-DBH), 4-hydroxybenzoic acid (4-HB), benzoic acid, vanillic acid and catechol [42].
Mahjoubi et al., showed that Stenotrophomonas maltophilia, isolated from petroleum contaminated sediments and seawater at the refinery harbor of the Bizerte coast, in the north of Tunisia, were the most active hydrocarbonoclatic isolated strain with significant production of biosurfactants and high emulsification activity of crude oil (up to 46%), and suggest by their results that Stenotrophomonas maltophilia may constitute a potential candidate for bioremediation, and could be useful for biotechnological applications [7]. It was demonstrated that this species had the ability to utilize a wide range of aromatic substrate as a sole carbon source, and could degrade 80% of phenanthrene in 48 hours comparing to Pseudomonas that degraded 70% of phenanthrene in 40 days [43,44]. Moreover, Stenotrophomonas maltophilia is a very powerful and useful means in the biotreatment of sole and wastewater decontamination [7].
The isolated strain from contaminated marine sediments and seawater in our study tolerate significant concentrations of NaCl (up to 15% w/v) and crude oil (up to 10% v/v) because of the polluted area and mechanisms of induction of developed enzymes of interest and this tolerance may reflect the evolutionary adaptation. Thus, strain SP54N may be a new species of Stenotrophomonas maltophilia. This adaptation translates in high stability which gives bacteria the ability to answer more quickly to a new source of hydrocarbons then bacteria without spoilage [45-48].
Conclusion
In the present study, a firstly isolated marine petroleum hydrocarbon-degrading bacterium strain SP54N, from mixture seawater and marine sediments of the Oran harbor-Algeria, was identified as Stenotrophomonas maltophilia according to its phenotypic and phylogenetic characteristics, possessed an outstanding growth capacity in crude oil, and excellent adaptability in pH and salinity, and could be used as a good degrader, to establish one eco-friendly, and cost-effective method for the removal of hydrocarbon contaminants in diverse marine environments polluted by petroleum hydrocarbons and crude oil, particularly in harbor of Oran.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgment
This study was funded by Laboratory of (AquaBior), Depart ment of Biotechnology, University of Oran 1 Ahmed Ben, Faculty of Nature and Life Sciences, Oran-Ministry of Higher Education and Scientific Research (Algeria). The authors thank the scientific group of Aquaculture and Bioremediation Laboratory (AquaBior) for their fruitful help for the development of this work.
Funding
This study was funded by the Laboratory of Aquaculture and Bioremediation (AquaBior) and by the Ministry of Higher Education and Scientific Research, Algeria.
References
- Deng MC, Li J, Liang FR, et al. Isolation and characterization of a novel hydrocarbon-degrading bacterium Achromobacter sp. HZ01 from the crude oil-contaminated seawater at the Daya Bay, southern China. Mar Pollut Bull. 2014;83(1):79-86.
- Jiang Z, Huang Y, Xu X, et al. Advance in the toxic effects of petroleum water accommodated fraction on marine plankton. Acta Ecologica Sinica. 2010;30(1):8-15.
- Gong Y, Zhao X, Cai Z, et al. A review of oil, dispersed oil and sediment interactions in the aquatic environment: influence on the fate, transport and remediation of oil spills. Mar Pollut Bull. 2014;79(1-2):16-33.
- Hassanshahian M, Emtiazi G, Cappello S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull. 2012;64(1):7-12.
- Cappello S, Caruso G, Zampino D, et al. Microbial community dynamics during assays of harbor oil spill bioremediation: a microscale simulation study. J Appl Microbiol. 2007;102(1):184-94.
- Hii YS, Law AT, Shazili NA, et al. Biodegradation of Tapis blended crude oil in marine sediment by a consortium of symbiotic bacteria. Int Biodeterior Biodegradation. 2009;63(2):142-50.
- Mahjoubi M, Jaouani A, Guesmi A, et al. Hydrocarbonoclastic bacteria isolated from petroleum contaminated sites in Tunisia: isolation, identification, and characterization of the biotechnological potential. New Biotechnol. 2013. 2013;30(6):723-33.
- Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour Technol. 2017;223:277-86.
- Lee RF, Anderson JW. The significance of cytochrome P450 system responses and levels of bile fluorescent aromatic compounds in marine wildlife following oil spills. Mar Pollut Bull. 2005;50(7):705-23.
- Song X, Xu Y, Li G, et al. Isolation, characterization of Rhodococcus sp. P14 capable of degrading high-molecular-weight polycyclic aromatic hydrocarbons and aliphatic hydrocarbons. Mar Pollut Bull. 2011;62:2122-28.
- Liu R, Zhang Y, Ding R, et al. Comparison of archaeal and bacterial community structures in heavily oil-contaminated and pristine soils. J Biosci Bbioeng. 2009;108(5):400-07.
- Atlas RM, Hazen T C. Oil biodegradation and bioremediation: A totale of the two worst spills in U.S. history. Environ Sci Technol. 2011;45:6709-15.
- Liu SH, Zeng GM, Niu QY, et al. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour Technol. 2017;224:25.
- Mehdi H, Giti E. Investigation of alkane biodegradation using the microtiter plate method and the correlation between biofilm formation, biosurfactant production, and crude oil biodegradation. Int Biodeterior Biodegradation. 2008;62(2):170-78.
- Holt SG, Kriey NR, Sneath PHA, et al. Bergy’s Manual of determination for Bacteriology. Williams and Wilkins, New York. 1998.
- Sievers F, Higgins DG. Clustal Omega, accurate alignment of very large numbers of sequences. In Multiple sequence alignment methods. Humana Press, Totowa, NJ. 2014;105-116.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870-04.
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406-25.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783-91.
- Neethu CS, Rahiman KM, Rosmine E, et al. Utilization of agro-industrial wastes for the production of lipase from Stenotrophomonas maltophilia isolated from Arctic and optimization of physical parameters. Biocataly Agric Biotechnol. 2015;4(4):703-09.
- Kumari S, Regar RK, Manickam N. Improved polycyclic aromatic hydrocarbon degradation in crude oil by individual and a consortium of bacteria. Bioresour Technol. 2018;254:174-79.
- Vandecasteele JP. Microbiologie pétrolière: Concepts implications environmentales applications industrilelles. Technip. 2005;1:534-35.
- Rouf R, Karaba SM, Dao J, et al. Stenotrophomonas maltophilia strains replicate and persist in the murine lung, but to significantly different degrees. Microbiology. 2011;157(7):2133-42.
- Rodríguez-Blanco A, Antoine V, Pelletier E, et al. Effects of temperature and fertilization on total vs. active bacterial communities exposed to crude and diesel oil pollution in NW Mediterranean Sea. Environ Pollut. 2010;158(3):663-73.
- Wang Y, Feng J, Lin Q, et al. Effects of crude oil contamination on soil physical and chemical properties in Momoge wetland of China. Chin Geogr Sci. 2013;23(6):708-15.
- Shiaris MP. Phenanthrene mineralization along a natural salinity gradient in an urban estuary, Boston Harbor, Massachusetts. Microb Ecol. 1989;18(2):135-46.
- Malkawi HI, Fatmi LM, Al-deeb TM. Mutational analysis of oil-degrading genes in bacterial isolates from oil contaminated soil at the Jordanian oil refinery. World Appl Sci J. 2009;6(2):208-20.
- Roder A, Hoffmann E, Hagemann M, et al. Synthesis of the compatible solutes glucosylglycerol and trehalose by salt-stressed cells of Stenotrophomonas strains. FEMS Microbiol Lett. 2005;243(1):219-26.
- Lee EY, Jun YS, Cho KS, et al. Degradation characteristics of toluene, benzene, ethylbenzene, and xylene by Stenotrophomonas maltophilia T3-c. J Air Waste Manage Assoc. 2002;52(4):400-06.
- Tiwari B, Manickam N, Kumari S, et al. Biodegradation and dissolution of polyaromatic hydrocarbons by Stenotrophomonas sp. Bioresour Technol. 2016;216:1102-05.
- Chen S, Yang L, Hu M, et al. Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Appl Microbiol Biotechnol. 2011;90(2):755-67.
- Tang H, Li J, Hu H, et al. A newly isolated strain of Stenotrophomonas sp. hydrolyzes acetamiprid, a synthetic insecticide. Process Biochem. 2012;47(12):1820-25.
- Kobayashi DY, Reedy RM, Bick J, et al. Characterization of a chitinase gene from Stenotrophomonas maltophilia strain 34S1 and its involvement in biological control. Appl Environ Microbiol. 2002;68(3):1047-54.
- Li Z, Nandakumar R, Madayiputhiya N, et al. Proteomic analysis of 17ß-estradiol degradation by Stenotrophomonas maltophilia. Environ Sci Technol. 2012;46(11):5947-55.
- Fang Z, Zhang J, Liu B, et al. Biochemical characterization of three keratinolytic enzymes from Stenotrophomonas maltophilia BBE11-1 for biodegrading keratin wastes. Int Biodeterior Biodegradation. 2013;82:166-72.
- Mukherjee P, Roy P. Genomic potential of Stenotrophomonas maltophilia in bioremediation with an assessment of its multifaceted role in our environment. Front Microbiol. 2016;7:967.
- Venkidusamy K, Megharaj M. Identification of electrode respiring, hydrocarbonoclastic bacterial strain Stenotrophomonas maltophilia MK2 highlights the untapped potential for environmental bioremediation. Front Microbiol. 2016;7:1965.
- Kumari S, Regar RK, Manickam N. Improved polycyclic aromatic hydrocarbon degradation in crude oil by individual and a consortium of bacteria. Bioresour Technol. 2018;254:174-9.
- Arulazhagan P, Vasudevan N, Yeom IT. Biodegradation of polycyclic aromatic hydrocarbon by a halotolerant bacterial consortium isolated from marine environment. Int J Environ Sci Tech. 2010;7(4):639-52.
- Gao S, Seo JS, Wang J, et al. Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. Int Biodeterior Biodegradation 2013;79:98-104.
- Shuona C, Hua Y, Jingjing C, et al. Physiology and bioprocess of a single cell of Stenotrophomonas maltophilia in bioremediation of co-existed benzo [a] pyrene and copper. J Hazard Mat. 2017;321:9-17.
- Urszula G, Izabela G, Danuta W, et al. Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Braz J Microbiol. 2009;40(2):285-91.
- Wojcieszynska D, Hupert-Kocurek K, Gren I, et al. High activity catechol 2, 3-dioxygenase from the cresols-Degrading Stenotrophomonas maltophilia strain KB2. Int Biodeterior Biodegradation. 2011;65(6):853-58.
- Kahlon RS. Biodegradation and bioremediation of organic chemical pollutants by Pseudomonas. In Pseudomonas. Mol Appl Biol. 2016;343-417.
- Girvan MS, Campbell CD, Killham K, et al. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ Microbiol. 2005;7(3):301-13.
- Maila MP, Randima P, Drønen K, et al. Soil microbial communities: Influence of geographic location and hydrocarbon pollutants. Soil Biol Biochem. 2006;38(2):303-10.
- Bordenave S, Goni-Urriza MS, Caumette P, et al. Effects of heavy fuel oil on the bacterial mat. Appl Environ Microbiol. 2007;73(19):6089-97.
- Molina M, Gonzalez N, Bautista L, et al. Isolation and genetic identification of PAH-degrading bacteria from the microbial consortium. Biodegradation. 2009;20(6):789-800.