Original Article
, Volume: 12( 2)Hepato-preventive Activity of Camel Milk Against CCl4-Induced Lesions in Mice
- *Correspondence:
- Gargouri M and Hamed H Laboratory of Animal Ecophysiology, Faculty of Sciences, University of Sfax, 3038 Sfax, Tunisia
Tel: + 216 74 241 888; Fax: + 216 74 246 217; E-mail: manele.gargouri@gmail.com
Received Date: May 8, 2017 Accepted Date: May 16, 2017 Published Date: May 24, 2017
Citation:Hamed H, Gargouri M, Bellassoued K, et al. Hepato-preventive Activity of Camel Milk Against CCl4-Induced Lesions In Mice. Res Rev Biosci. 2017;12(2):117.
Abstract
This study was aimed at evaluating the potential effects of a mice exposure to CCl4 single dose on liver tissue and potent preventive effects of camel milk (CM) orally treated by gavage. Additionally, CCl4 was administered by intra-peretonial injection at day 14. Administration of CCl4 caused an increase in lipid peroxidation and a decrease in superoxide dismutase, catalase, glutathione peroxidase activities and GSH levels in liver. Moreover, CCl4 caused a distinguished rise of liver level markers (AST, ALT, ALP, LDH, ɤ-GT), TG, TC, and LDL-Ch but a reduced levels of HDL-Ch. In contrast, no such harms or biochemical changes were found in mice treated with camel milk against typically observed changes in CCl4-treated animals. These findings strongly prove that beneficial effects of Camel Milk clearly shown through the reduction of the CCl4 induced related damages and oxidative stress.
Keywords
Carbon tetrachloride; Camel milk; Hepatoprotection; Histopathological studies; Mice; Oxidative stress
Abbreviations
AST: Aspartate Transaminase; ALT: Alanine Transaminase; ALP: Alkaline Phosphatases; CAT: Catalase; CM: Camel Milk; ɤ-GT: ɤ-Glutamyl Transpeptidase; GPx: Glutathione Peroxidase; GSH: Reduced GSH; HDL-Ch: High density lipoproteins of cholesterol; LDH: Lactate Dehydrogenase; LDL-Ch: Low density lipoproteins of cholesterol; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; TBARS: Thiobarbituric Acid Reactive Substances; T-Ch: Total Cholesterol; TG: Triglycerides
Introduction
Liver diseases remain one of the major threats to public health and a worldwide problem [1]. The World health organization estimates that 46% of global disease and 59% of mortality are due to chronic disease [2] and the management of liver disease remains a significant concern of modern medicine since this latter has little to offer as alleviation of hepatic ailments [3]. Liver is the main organ, which is responsible for several metabolic functions. It is the organ in charge of many important life functions, including food digestion, glycogen storage, control of metabolism, drug detoxification and hormone production [4]. Since liver is so vital to life, malfunction or failure of the organ often results in high rates of morbidity and mortality. Various substances are known to cause liver damage, and one of them is carbon tetrachloride (CCl4), which is a well-known hepatotoxin [5]. Within the body, CCl4 breaks down to highly toxic trichloromethyl and trichloromethyl peroxyl free radicals by cytochrome P450 enzyme [6] which cause damage to hepatocytes and acts as alterations in biochemical, hematologic parameters [7]. The hepatotoxic effect of CCl4 is partially circumvented by antioxidant compounds including vitamins C and E as non-enzymatic antioxidants [8] and silymarin [9]. Induction of liver injury by CCl4 has been used vastly as a model for investigation of hepato-protective agents [8-11]. When the liver is injured, its treatment with famous chemical drugs remains limited [12]. Therefore, interest in the benefits of alternative medicines as a treatment of hepatic disease has been arisen. The richness of camel’s milk in peptides and proteins exhibits its biological activities that have beneficial effect on many bioprocesses as digestion, absorption, growth and immunity [13,14].
Furthermore, camel’s milk can be conserved at room temperature longer period than other animals’ milk [15]. The most known uses of camel’s milk are as drug against autoimmune diseases, dropsy, jaundice, splenomegaly, tuberculosis, asthma, anemia, piles and diabetes [16]. Also, camel’s milk is characterized by antitoxic effect in the face of 4 cadmium chloride [17,18], CCl4 [19], Cisplatin [20], Paracetamol [21], Aluminum chloride [22]. Although, Althnaian et al. [23] confirmed that Camel milk had a protective effect against CCl4 induced hepatotoxicity. As far as we know, such protective effect on antioxidant enzymes (CAT, SOD, GPx activities, lipoprotein profiles and GSH levels) has not yet been elucidated. Hence, the present investigation focus on evaluating the hepato-preventive potential of camel milk in CCl4- in vivo- affects antioxidant enzyme activities and lipid peroxidation indicating liver damage in mices.
Methods and Materials
Milk sampling
Milk used in this study was collected from dromedary animals (Camelus dromedaries) of Maghrabi breed from the south of Tunisia. The dromedaries were fed throughout the year exclusively by grazing. Dromedary sample was obtained by manual milking, collected into sterile bottles and transferred immediately to the laboratory.
Animals
Mice weighing 29 to 32 g purchased from Central Pharmacy (SIPHAT, Tunisia). Animals were kept in an air-conditioned room (temperature 21 ± 1°C and relative humidity of 40%) with a 12h light/dark cycle. All mice had free access to drinking water and diet. The pelleted diet for rats was 15% protein and supplied by the Industrial Society of Concentrate (SICO, Sfax, Tunisia). According to the general guidelines on the use of living animals in scientific investigations, the experimental procedures were carried [24] and approved by the Ethical Committee of the Faculty of Science of Sfax.
Reagents
The present study used reagents of analytical grade. Carbon tetrachloride was obtained from SD Fine Chemicals, Bhoisar, Mumbai, India. 5,5’-Dithiobis (2-nitrobenzoic acid) (DTNB), LGlutathione (reduced form), and all current chemicals were purchased from Sigma Chemical Co., (St. Louis, MO, USA).
Enzymes kits
At the end of experiment, blood samples were collected with heparin aortic puncture of dams. They were centrifuged at 3000 rpm for 20 min. Plasmatic activities of transaminases, i.e., alanine aminotransferase (ALT) and aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and ɤ-Glutamyl transpeptidase (ɤ-GT) kits were purchased from Bioerieux Laboratory. Triglycerides (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-Ch) and highdensity lipoprotein cholesterol (HDL-Ch) were obtained from the Beckman Coulter Laboratory (Brea, CA, USA).
In vitro study
Determination of antioxidant activities
Total antioxidant capacity, DPPH* radical-scavenging and reducing power inhibition were successively measured to evaluate the antioxidant activity of fermented milk and rosemary extracts.
Determination of total antioxidant capacity
The assay is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of a green phosphate/Mo5+ complex at an acid pH [25]. An aliquot (0.1 mL) of diluted samples was combined with 1 mL of reagent solution. Methanol was used instead of sample for the blank. After being incubated in a boiling water bath for at 95°C for 90 min. After the mixture had cooled to room temperature, the mean of the absorbance values was measured at 695 nm against a blank. The antioxidant activity was expressed as the number of equivalents of ascorbic acid t (mg ascorbic acid equivalent [AA] g−1 dry weight [DW]).
DPPH de-coloration assay
The antioxidant activities of the camel milk using the DPPH method of Hanato et al. [26]. Briefly, 1 mL of various extract concentrations (1-40 μg mL−1) was added to 250 μL of 0.2 mmol/L DPPH methanol solution. After 30 minutes at room temperature in the dark. The absorbance of the resulting solution was then read 517 nm and butylated hydroxytoluene as a standard. The ability to scavenge the DPPH radical was calculated using the following equation:
DPPH• scavenging effect (%) = (A0–A1/ A0) × 100
where A0 and A1 are the absorbance at 30 minutes of the control at 30 min and A1 is the absorbance of the sample at 30 min. All samples were analyzed in triplicate.
Ferric reducing power activity methods
The FRAP procedure was performed as described by Megías et al. [27]. Briefly, a proper amount of sample was added with 0.9 mL of reagent (0.83 mmol (TPTZ, 2,4,6-Tripyridyl-sTriazine) and 1.67 mmol ferric chloride in 0.1 mol acetate buffer (pH 3.6) and incubated at 25°C for 10 min. The reduction of Fe3+ -TPTZ to Fe2+-TPTZ was monitored by the absorbance increase at 595 nm. Trolox was used as a positive control. Measurements were done with each sample in triplicates.
in vivo study
Experimental design
Twenty-eight mice were randomly assigned to groups of seven animals each. group (C): control mice received distilled water; group CCl4: (a CCl4 hepatotoxicity pathological model) was given a single dose of CCl4 (10 ml/kg in 0.3% olive oil. ip) at day 14; group CM: daily administrated by oral gavage 0.4 mL of camel milk during 15 day; group CM+CCl4: pretreated with camel milk and intoxicated with CCl4 at 14 day. During the period of treatment, all animals survived and weighed every day.
Organ sampling
At the end of the experiment period, control and treated mice after anesthesia by intraabdominal injection with chloral hydrate. Blood samples were collected with heparin from brachial artery of animals. They were centrifuged at 2500 g for 15 minutes, and stocked at −20°C until analysis. Liver were dissected, cleaned and weighed. Some samples were homogenized (1:2, w/v) in 50 mmol/L Tris buffer (pH 7.4) containing 150 mmol/L NaCl using an ultra-Turrax device. Some samples were rinsed and homogenized at centrifuged at 5000g for 25 min at 4°C and aliquots of supernatant were kept at −30°C until analyses. In parallel, portions of liver and other organs were immediately fixed into Bouin solution (saturated picric acid added with 37% to 40% formaldehyde and glacial acetic acid, 75:25:5 v/v) for histological studies [28].
Biochemical parameters
Protein determination in liver
Liver protein content contents were measured according to Bradford [29] using bovine serum albumin (BSA) as a standard.
Evaluating antioxidant enzyme and lipid peroxidation level
Levels of lipid peroxidation was estimated in liver by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Yagi [30]. Catalase (CAT), Superoxide dismutase (SOD), and glutathione-peroxidase (GPx) activities were determined in the same extracts according to the methods of Aebi [31], Beyer and Fridovich [32], and Flohe and Gunzler [33] respectively.
Glutathione content in liver
The contents of GSH was estimated by using the method of Ellman [34] after reaction with 5,50-dithiobis-(2-nitrobenzoic acid).
Histopathological examination
Specimens from liver tissues were taken from all rat groups after scarification. They collected and fixed in bouin solution and embedded into paraffin, cut at 2- 5 μm slices and stained with hematoxylin and eosin for histological examination [35]. Eights slices from each liver were prepared.
Statistical analysis
Statistical analyses were performed using one-way ANOVA followed by Fisher's post-hoc test (PLSD) for comparison between groups [treated groups (CCl4 vs (C)] and [(CM+ CCl4,) vs (CCl4)]. Data are expressed as mean ± SE. Differences were considered significant if (P<0.05, P<0.01, P<0.001). Power analysis was performed upon each parameter measurement to determine sample size.
Results
Impacts of CCl4 on health Death or abortion did not occur in any experimental groups during the treatment period (15 days). However, few clinical symptoms were noted in mice, including decreased activity and apparent weakness.
Antioxidant activities of camel milk
It is obvious from Table 1, that the camel milk sample had a high ability to scavenge DPPH*radical (IC50=0.63 mg/ml). It can be shown that a high ferrous ion chelating activity of CM. In addition, it can be seen that CM reveal a significant increase in their total antioxidant capacity (0.96 mg AAE/ml). Effect of treatments on serum transaminase, PAL, LDH and ɤ-GT activities Table 2 shows that CCl4 had significantly raised serum ALT, AST, PAL, LDH and ɤ-GT levels in mice liver as compared to control (+212%, P<0.001; +75%, P<0.01; +34%, P<0.01; +72%, P<0.01; +109%, P<0.001, respectively) (Table 2). Treatment with CM alone didn’t cause any effect per se on the hepatic biomarkers in mice. Pretreatment with camel milk for 15 days restored ALT, AST, PAL, LDH and ɤ-GT (P<0.01), without attain normal values.
Values represent the means ± SE of 3 replicates.
| Antioxidant activities | |||
|---|---|---|---|
| Camel milk | TACa | DPPHb | FRAPc |
| 0.96 ± 0.05 | 0.63 ± 0.02 | 2.55 ± 0.02 | |
Table 1. Antioxidant activities in the camel milk
| Hepatic Biomarkers | Groups =7 | |||
|---|---|---|---|---|
| C | CM | CCl4 | CCl4+CM | |
| AST1 (U/L) | 120 ± 12.5 | 130 ± 12 | 211 ± 13.8*** | 124.4 ± 13### |
| ALT2 (U/L) | 6.41 ± 1.7 | 6.21 ± 1.9 | 20.00 ± 3.3*** | 9.61 ± 2.1### |
| ALP3 (U/L) | 79.00 ± 8.2 | 82.00 ± 8 | 116 ± 11*** | 88 ± 7.3## |
| LDH4 (U/L) | 2283 ± 112 | 2290 ± 99 | 4000 ± 108*** | 2684 ± 97### |
| ɤ-GT5 (U/L) | 1.10 ± 0.70 | 1.19 ± 0.60 | 2.30 ± 0.10*** | 1.8 ± 0.20## |
C: Control; CM: Camel milk; CCl4: Carbon tetrachloride; CCl4+ CM: Mice pre-treated with camel milk and intoxicated with CCl4 at 14th day
1AST, Aspartate transaminase; 2ALT, Alanine transaminase; 3ALP, Alkaline phosphatases; 4LDH, lactate dehydrogenase; 5ɤ-GT. Values are expressed as means ± SE of seven samples per group.
One-way ANOVA followed by Fisher's protected least significant difference (PLSD) as a post hoc test for comparison between groups:
Comparison between (CCl4) vs. control (C) group: * P<0.05; ** P<0.01; *** P<0.001.
Comparison between [CM + CCl4] groups vs. (CCl4) group: # P<0.05; ## P<0.01; ### P<0.001. ɤ-Glutamyl transpeptidase
Table 2. Hepatic biomarkers (AST ALT, ALP, LDH and ɤ-GT) levels in mice plasma at 15 days of treatment with camel milk.
A. Total antioxidant capacity (mg AAE g−1 DW).
B. DPPH scavenging activity (mg mL−1).
C. FRAP (μg mL−1).
Effects of camel milk on lipid profile
CCl4 led to severe metabolic changes in mice liver, as shown by a significant increase in triglycerides (TG) (+121%), total cholesterol (TC) (+43%) and LDL-Ch (+76%) levels, as well as decreased HDL levels (-49%) (Table 3). Treatment with CM did not cause any significant change in lipid profile in liver. Pretreatment with camel milk for 15 days restored TG, TC and LDL-Ch levels (P<0.01), without reaching normal values. Conversely, HDL-Ch level showed a significant increase as comparted to CCl4 group. Estimation of lipid peroxidation in liver The level of TBARS were revealed an increase in liver tissue of the CCl4 treated animals compared to control untreated mice (+151%, P <0.001) (Table 4). Here again, treatment of CM alone didn’t cause any effect per se on the lipid peroxidation level in its mice. The use of camel milk relieves lipid peroxidation without attaining normal values (Table 4).
| Parameters | Groups n=7 | |||
|---|---|---|---|---|
| C | CM | CCl4 | CCl4+CM | |
| 1TG (mg/L) | 1.05 ± 0.12 | 1.12 ± 0.02 | 2.33 ± 0.25*** | 1.40 ± 0.13# |
| 2T-Ch (mg/L) | 1.90 ± 0.15 | 1.97 ± 0.11** | 3.70 ± 0.25** | 2.10 ± 0.15## |
| 3HDL (mg/L) | 1.94 ± 0.15 | 1.92 ± 0.19 | 0.98 ± 0.09*** | 1.82 ± 0.30### |
| 4LDL (mg/L) | 0.6 ± 0.03 | 0.51 ± 0.06 | 1.06 ± 0.10** | 0.79 ± 0.17## |
C: Control; CM: camel milk; CCl4: Carbon tetrachloride; CCl4+ CM: Mice pre-treated with camel milk and intoxicated with CCl4 at 14th day.
1TG, Triglycerides; 2T-Ch, Total cholesterol; 3HDL-Ch, High density lipoproteins of cholesterol; 4LDL-Ch, Low density lipoproteins of cholesterol. Values are expressed as means ± SE of seven samples per group.
One-way ANOVA followed by Fisher's protected least significant difference (PLSD) as a post hoc test for comparison between groups:
Comparison between (CCl4) vs. control (C) group: * P<0.05; ** P<0.01; *** P<0.001.
Comparison between [CM+ CCl4,] groups vs. (CCl4) group: #P<0.05; ## P<0.01; ### P<0.001.
Table 3. Serum triglycerides, cholesterol, HDL-Ch and LDL-Ch levels (in mg/L) in mice at 15 days of treatment with camel milk.
| Parameters | Groups =7 | |||
|---|---|---|---|---|
| C | CM | CCl4 | CCl4+CM | |
| 1TBARS | 1.38 ± 0.09 | 1.42 ± 0.01 | 3.47 ± 0.50*** | 1.97 ± 0.30## |
| 2SOD | 137.2 ± 11 | 140.4 ± 10 | 94.95 ± 10** | 110.69 ± 22# |
| 3CAT | 0.86 ± 0.03 | 1.05 ± 0.06 | 0.14 ± 0.09*** | 0.73 ± 0.006## |
| 4GPx | 4.52 ± 0.10 | 4.37 ± 0.30 | 1.92 ± 0.20*** | 3.66 ± 0.20## |
| 5GSH | 0.77 ± 0.002 | 0.66 ± 0.028 | 0.16 ± 0.03*** | 0.52 ± 0.015## |
C: Control; CM: camel milk; CCl4: Carbon tetrachloride; CCl4+ CM: Mice pre-treated with camel milk and intoxicated with CCl4 at 14th day.
1TBARS, Thiobarbituric acid reactive substances (nmol/mg protein); 2SOD, Superoxide dismutase (U SOD/mg protein); 3CAT, Catalase (nmol/mg
protein); 4GPx, Glutathione peroxidase (nmol/mg protein); 5GSH, Reduced GSH
Values are expressed as means ± SE of seven samples per group. One-way ANOVA followed by Fisher's protected least significant difference (PLSD) as a post hoc test for comparison between groups:
Comparison between (CCl4) vs. control (C) group: * P<0.05; ** P<0.01; *** P<0.001.
Comparison between [CM + CCl4] groups vs (CCl4) group: # P<0.05; ## P<0.01; ###P<0.001.
Table 4. Effect of camel milk on oxidative status and antioxidant system activity in mice liver.
Estimation of GSH levels in the liver
noticed decrease of GSH level in the liver was evident in the CCl4 group (3-fold, P<.001), compared with those of controls (Table 4). Pretreatment with camel milk ameliorated GSH level compared with the CCl4 group.
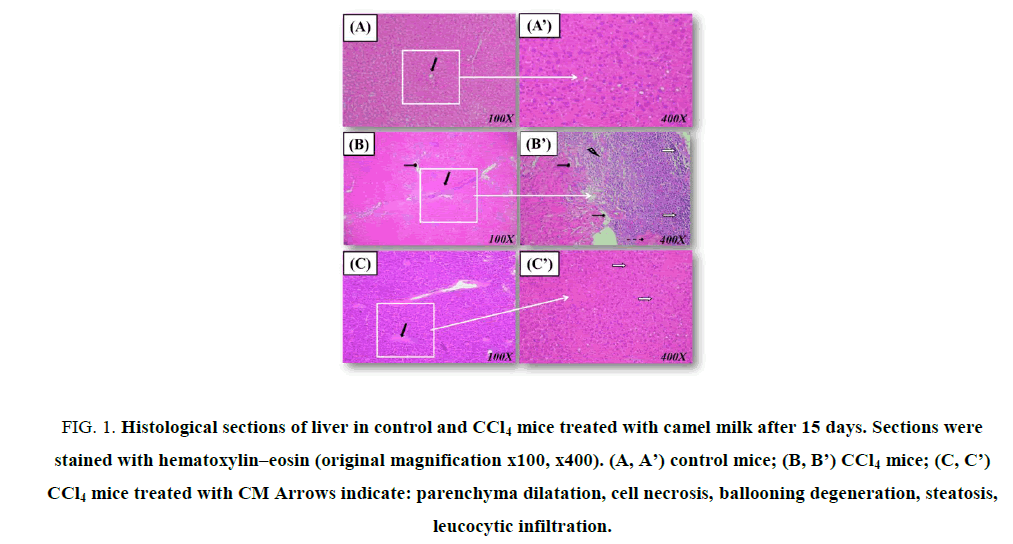
Enzymatic antioxidant status in the liver In the liver homogenates of CCl4-treated mice, SOD, CAT and GPx activities decreased significantly by -44.3%, - 5 fold, and -1.3 fold respectively, when compared to controls (Table 4). Pretreatment camel milk at 0.4 mL improved these parameters when compared to the CCl4 group without reaching normal values. Histological studies The biochemical modifications mentioned above were connected with our histological studies (Figure. 1). In fact, in the liver sections of control group showed normal hepatic cells [Figure. 1. (A, A’)]. The administration of CCl4 caused severe hepatocytes necrosis, inflammation and hepatocyte ballooning [Figure. 1. (B, B’)]. Pretreatment with camel milk was orally administrated of CCl4-treated mice [Figure. 1. (C, C’)], the liver sections’ histological aspects were partially reversed compared to the control group. The histological aspects of the liver of the camel milk treated group were similar to those of controls (data not show).
Figure 1: Histological sections of liver in control and CCl4 mice treated with camel milk after 15 days. Sections were stained with hematoxylin–eosin (original magnification x100, x400). (A, A’) control mice; (B, B’) CCl4 mice; (C, C’) CCl4 mice treated with CM Arrows indicate: parenchyma dilatation, cell necrosis, ballooning degeneration, steatosis, leucocytic infiltration.
Discussion
Liver fibrosis represents chronic wound repair following liver injury [36]. In this study, we assessed the response of the liver to hepatotoxicants in mice with a preceding liver injury. First, in our study, hepatotoxicity of the CCl4 in the mice was determined by changes in serum parameters. Examination of liver was done through the estimation of the activities of serum ALT, AST, ALP LDH and ɤ-GT which are enzymes originally present at elevated concentration in the cytoplasm [37-40]. In case of hepatopathy, these enzymes escape into the blood stream in accordance with the extent of liver damage [41,42]. Our results showed that administration of CCl4 induced a significant elevation of enzyme levels in comparison with normal control. The elevated level of enzymes such as aspartate aminotransferase and alanine amino transferase have been seen in rats received CCl4 leading to increased cell damage, permeability, and hepatocytes necrosis [43-44].
Alkaline phosphatase as a membrane bound enzyme, is excreted in association with bile when liver is affected, defective excretion increased the serum level of this enzyme [45]. Second, when reactive oxygen species attack polyunsaturated fatty acids, lipid peroxidation products are constitute causing a membrane structural and/or functional damage [46], manifested by an elevation in liver fibrosis induced by CCl4 [47]. Indeed, the present study show an elevation of lipid peroxidation at the level liver of mice treated with CCl4, leading to tissue damage and failure of antioxidant defense mechanism to prevent the formation of excessive free radicals [48-50]. Noteworthy, preventive treatment with camel milk was proved to reduce the rise of serum AST and ALT activities induced by CCl4 treatment in rats. This finding implies that camel milk has the challenge to protect liver tissue from CCl4 injury. The increased of serum enzymes caused by CCl4 induced liver damage. Camel milk through its ability of the leakage of intracellular enzymes may be to the prevention by its membrane stabilizing activity. This is in agreement with the commonly accepted view that serum levels of transaminases return to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes [51].
Many researchers have provided a significant support for proving camel milk protective effects on liver damage [19,52,53]. In addition, these studies insisted that the protective effect of camel milk against CCl4-induced oxidative stress in the rat is owing to its antioxidant properties. Camel milk contains high concentrations of vitamins A, B2, C and E and is very rich in magnesium and other trace elements; these vitamins act as antioxidants and have been found to be beneficial in preventing toxicant-induced tissue injury [54]. The efficiency of any hepatoprotective drug is based on its ability of either reducing the harmful effect or restoring the normal hepatic physiology that has been distributed by a hepatotoxin. Camel milk reduced CCl4 induced elevated enzyme levels in tested groups, indicating the protection of structural integrity of hepatocytic cell membrane or regeneration of damaged liver cells [55]. Second, preventive treatment of CM can repair and protect liver tissues by stabilizing the membrane and the prevention of intracellular enzyme leakages. It could be concluded that the reversal of serum transaminase levels is owing to the repair and healing process of hepatic parenchymal cells [56]. CM impacts had been reported previously in several related topics [57,58], that attribute the harmful hepatic effects restoration, back to normal physiology; to CM consumption which affects the regeneration or protection of hepatocyte membrane integrity [59].
Third, this research has also proved that, the CCl4 treatment could have an impact on the lipid metabolism of liver (triglyceride and cholesterol levels). This is concluded from the present observations that, CCl4 caused a significant (P <.0.05) increase in the levels of lipid parameters. Althnaian et al. [60] confirmed that CCl4 intoxication resembles to hepatitis in case of the triglycerides catabolism. Moreover, it can be assumed that hypercholesterolemia in CCl4 intoxicated mice was the consequence of the damage of hepatic parenchymal cells that lead to disturbance of lipid metabolism in liver [61]. However, the beneficial effects of camel milk on lipid metabolism have not been studied well. Our data have showed a significant decline in triacylglycerol cholesterol, LDL-C values but it increased the HDL-C levels compared to CCl4-intoxicated mice. Lipid lowering is the main effect of camel milk which might be attributed to an inhibitory activity on microsomal acyl coenzyme A: cholesterol acyltransferease in vitro. This latter is responsible for acylation of cholesterol-to cholesterol esters in liver [61].
Conclusion
In conclusion, CCl4 treatment resulted in hepatic lesions with hepatic, oxidative damage and histopathological changes. Pretreatment with camel milk led to a significant attenuation of hepatic disorders. Our results suggested that pretreatment with camel milk could be a useful adjunct in order to avoid toxicity induced by CCl4. Further studies are needed to discover new pharmacological approaches and to determine the exact mechanistic pathways of camel milk.
Acknowledgments
The present work was supported by DGRST grant (Direction Générale de la Recherche Scientifique et Technique-Tunisie (Appui à la Recherche Universitaire de base UR/13 ES-73).
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plants Res. 2010;4: 1318-34.
- Williams R. Global challenges in liver disease. J Hepatology. 2006;44:21-6.
- Wagh AE, Yeotkar US, Nimbhorker MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Orient Phann. 2010;10:111-5.
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. J Dev Cell. 2010;18:175-89.
- Gnanaprakash K, Madhusudhana CC, Ramkanth S, et a1. J Int J Biollife Sci. 2010;6:51-5.
- Khan MR, Rizvi W, Khan GN, et al. Carbon tetrachloride-induced nephrotoxicity in rats: protective role of Digera muricata. J Ethnopharmacol. 2009;122:91- 9.
- Girish C, Koner BC, Jayanthi S, et al. J Indian J Exp Biol. 2009;47:257263.
- Fridovich I, Ann NY. Acad Sci. 1999; 893:13-8.
- Mourelle M, Muriel P, Favari L, et al. Prevention of CCl4-induced liver cirrhosis by silymarin. J Fundamental & Clinical Pharmacology. 1989;3:183-91.
- Naziroğlu M, Cay M, Ustündağ B, et al. Comparative evaluation of some flavonoids and tocopherol acetate against the systemic toxicity induced by sulphur mustard. J Cell Biochemistry and Function. 1999;17:253–59.
- Kanter M, Coskun O, Budancamanak M. Improvement analysis of article quality in World Journal of Gastroenterology during 2008-2012. J World Journal of Gastroenterology. 2005;11:6684-5.
- Lee CH, Park SW, Kim YS, et al. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. J Biol Pharm Bull. 2007;30:1898-1904.
- Yagil R, Saran A, Etzion Z, J Comp Biochem Physiol. 1984;78:263-66.
- Korhonen H, Pihlanto A. Food-derived bioactive peptides-opportunities for designing future foods. J Curr Pharm Des. 2001;9:1297-1308.
- Omer RH, Eltinay AH. J Pak J Nutr. 2009;8:607-10.
- Rao MB, Gupta RC. J Indian J Dairy Sci. 1970;23:71–78.
- Al-Hashem F. S-Allyl-cysteines reduce amelioration of aluminum induced toxicity in rats. J Am J Biochem Biotechnol. 2009;5:98-108.
- Dallak M. Transdermal fentanyl: pharmacology and toxicology. J Am J Pharmacol Toxicol. 2009;4:134-41.
- Khan A, Alzohairy A. Hepatoprotective effects of camel milk against CCl4-induced hepatotoxicity in rats. J Asian J Biochem. 2011;6:171-180.
- Afifi MEM. J Am J Biochem Biotechnol. 2010;6:141-47.
- Al-Fartosi KG, Majid A, Auda AM, et al. Biochemical and molecular investigation of antioxidant enzymes in liver tissue of rats intoxicated with carbon tetrachloride and treated with aqueous extract of fenugreek (Trigonella foenum-graecum L.). J Int J Res Pharmaceut Biomed Sci. 2012;3:385-9.
- Al-Hashem F. J Am J Biochem Biotechnol. 2009;5:98-108.
- Althnaian T. J Global Veterinaria. 2012;9:564-570.
- Council of European Communities. Council instructions about the protection of living animals used in scientific investigations. Official J Eur Communities (JO 86/609/CEE) 1986;L358:1-18.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. J Anal Biochem. 1999;269:337–41.
- Hatano T, Kagawa H, Yasuhara T, et al. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. J Chem Pharm Bull. 1988;36:2090-7.
- Megías C, Pastor-Cavada E, Torres-Fuentes C, et al. Influence of roasting treatment on the antioxidant activities and color of burdock root tea. J European Food Research and Technology. 2009;230:353-59.
- Gargouri M, Ghorbel-Koubaa F, Bonenfant-Magné M, et al. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. J Food Chem Toxicol. 2012;50:2303-10.
- Bradford MMA. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J Anal Biochem. 1976;72:248-54.
- Yagi KA. A simple fluorometric assay for lipoperoxide in blood plasma. J Biochem Med. 1976;15:21-6.
- Catalase AH. J New York Academic Press. 1974;67-84.
- Beyer WF, Fridovich I. J Anal Biochem. 1987;161:559-66.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. J Methods Enzymol. 1984;105:114-21.
- Ellman GL. Tissue sulfhydryl groups. J Arch Biochem Biophys. 1959;82:70-77.
- Gabe M, Masson. J Techniques Histologiques. Paris, Masson. 1968;838-41.
- Friedman S1. J Asian. J Exp Biol Sci. 2010;1:614-23.
- Kumawat R, Sharma S, Vasudeva N, et al. J Asian Pac J Trop. Biomed.2012;2:S947-S952.
- Hurkadale PJ, Shelar PA, Palled SG, et al. J Asian Pac J Trop Biomed. 2012;2:S238-S242.
- Rao BG, Rao YV, Rao TM. J Asian Pac J Trop Dis. 2012;2:S208-S211.
- Nkosi CZ, Opoku AR, Terblanche SE. J Phytother Res. 2005;19:341-345.
- Dominic A, malraj A, Parkavi C, et al. J Asian J Phann Technol. 2012;2:51-53.
- Battu GR, Venkateswara Rao Y, Dasari PVS. J Recent Res Sci Technol. 2012;4:21-24.
- Shahid SM, Shamim S, Mahboob T. J African J Phann Phannacol. 2012;6:1958-63.
- Nemesanszky E. J Enzyme Tests in Diagnosis, London. Arnold. 1996;25-59.
- Yoshida Y, Umeno A, Shichiri M. J Clin Biochem Nutr. 2013;52:9-16.
- Al-Sayed M, El-Lakkany NM, Seif El-Din SH, et al. J Pharm Biol. 2014;52:1581-90.
- Gasparovic AC, Jaganjac M, Mihaljevic B, et al. J Methods Mol Biol. 2013;965:283-296.
- Dinischiotu A, Stanca L, Gradinaru D, et al. J Methods Mol Biol. 2013;1028:155-64.
- Garba SH, Prasad J. J Biol Sci. 2007;7:276-81.
- Gaya M, Repetto V, Toneatto J, et al. J Biochim Biophys Acta. 2013;3796-3806.
- Hamad EM, Abdel-Rahim EA, Romeih EA. J International Journal of Dairy Science. 2011;6:190-97.
- Al-Fartosi KG, Majid A, Auda MA, et al. J Int J Res Pharmaceut Biomed Sci. 2012;3:385-89.
- Yousef MI. Toxicol. 2004;199:47-57.
- Palanive MG, Rajkapoor B, Kumar RS, et al. J Sci Pharm. 2008;76:203-15.
- Khan AA, Alzohairy M. J Asian J Biochem. 2011;6:171-80.
- Tong LM, Sasaki S, Julian M. J Agric Food Chem. 2000;48:1473- 78.
- Marcason W. J Am Diet Assoc. 2007;107:525–31.
- Wunjuntuk K, Kettawan A, Charoenkiatkul S, et al. J Med Food. 2016;1:15-23.
- Althnaian T, Albokhadaim I, El-Bahr SM. J Springer plus. 2013;2:57.
- Havel RJ. J Ann Rev Physio. 1986;48:119–34.
- Matsuda K. J Med Res Rev. 1994;14:271–305.