Original Article
, Volume: 12( 1)Head Space Solid Phase Micro-Extraction (HS-SPME) Gas Chromatography Mass Spectrometric (GC-MS) Analysis of Vinasse from Razi Alcohol Industry Using DVB/CAR/PDMS Fiber
- *Correspondence:
- Zahra Ramezani Toxicology Research Center, Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Tel: +98-6133738380; E-mail: zramezani@ajums.ac.ir
Received Date: January 19, 2018 Accepted Date: January 23, 2018 Published Date: January 29, 2018
Citation: Palash F, Ramezani Z, Rahbar N, et al. Head Space Solid Phase Micro-Extraction (HS-SPME) Gas Chromatography Mass Spectrometric (GC-MS) Analysis of Vinasse from Razi Alcohol Industry Using DVB/CAR/PDMS Fiber. Biochem Ind J. 2018;12(1):125
Abstract
Vinasse is among the byproducts of alcohol production factories. In this research, the aim has been to identify the volatile compounds present in vinasse, which are the cause of a stinking odor. Head space solid phase microextraction using PDMS/CAR/DVB commercial fiber as sorbent is used for absorption of the volatile compounds. The PDMS/CAR/DVB fiber was exposed to the headspace above the sample for 30 min. thereafter, the fiber was transferred to the injection port ofgass chromatograph (GC), and its components were separated. 32 separated compounds on the GC equipped with mass detector with a frequency percentage of over 0.9% were determined by the instrument library and by comparing their Kovat’s index in the literature. Among the major compounds in vinasse were ethanol gasoline (26.52%), 2-hydroxy benzoic acid (6.49%), decanoic acid ethyl ester (5.32%), and Limonene (4.02%). Therefore it was concluded that the present headspace micro extraction is well able to separate and detect the volatile compounds present in vinasse. It was found that eight of the detected compounds generate the stinking odor of vinasse.
Keywords
Vinass; Gas chromatography/Mass detector; Solid phase headspace micro-extraction; Alcohol production industries
Introduction
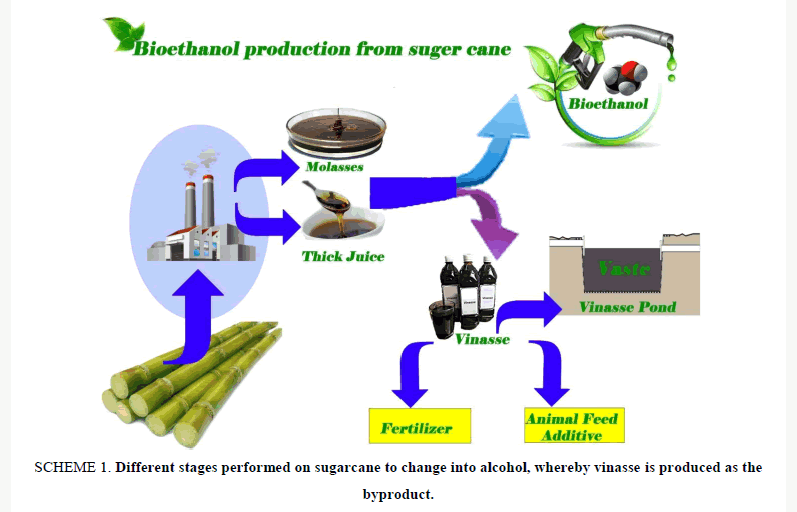
In the process of alcohol production, out of Beet molasses and sugar cane, using pre-concentration system, a compound called vinasse remains. Indeed, vinasse refers to the waste of alcohol production industries, which remains after distillation of alcohol. On average, per production of every litter of alcohol, 12 L vinasse is produced [1]. Vinasse is a dark brown compound with the odor of burnt sugar, which is rich in potassium, calcium, magnesium and some nitrogen and phosphorus.
In Latin American countries, consumption of vinasse in sugarcane farms with a light texture has caused increased performance. As vinasse is rich in potassium, if it is added to soil, it can enhance the absorbable potassium of soil. The vinasse of beetroot and sugarcane is widely used across different industries including feed of livestock and poultry, production of chemical and organic fertilizers, food industries, chemical industries and pharmaceuticals [2]. Nevertheless, its application as agricultural fertilizer and feed of animals is common in Brazil, though it has numerous problems such as the odor it develops in farms, which should be solved.
Vinasse also shows other effects including insecticide, which can have an inhibitory effect in agriculture, causing biological removal of agricultural pests (Scheme 1). Vinasse can also be used as a fertilizer or supplement along with cake filter. Vinasse has also been used in the production of biogas [2-5].
Scheme 1: Different stages performed on sugarcane to change into alcohol, whereby vinasse is produced as the byproduct.
In spite of the advantages of vinasse, if it is liberated in the nature or stored improperly, it can cause creation and emission of undesirable odors and subsequently complaints of residence especially in the downstream of wind due to degradation or evaporation of volatile organic compounds and part of inorganic gas along with it. Therefore, one of the problems of vinasse is production of unfavorable odor, which is especially annoying during conditions of environmental temperature elevation. In order to be able to remove this odor, first the volatile compounds in vinasse should be detected precisely.
In order to solve the problem of vinasse and identify its useful compounds as well as prevent unfavorable odor emission in the environment, several methods can be conducted. However, in all methods, the compounds exist in the vapors emitted from vinasse lagoon should be determined and characterized. The best method for detection of these compounds is the use of mass spectroscopy, therefore gas chromatography/mass spectrometry technique (GC/MS) is the best approach to separate and detect its volatile compounds [6,7]. Some studies have been conducted for determining the compounds present in solid vinasse using gas chromatography mass spectrometry technique and numerous organic compounds have been introduced [8]. In these studies, the vinasse water has been evaporated and the powdered sample has been characterized following extraction through different methods. Head space micro extraction technique is a quick method, with no need for sample preparation, which is able to determine volatile compounds of vinasse under its environmental conditions.
Extreme meteorological conditions affect the type and level of compounds. Furthermore, as the conducted studies indicate, no research has been conducted on the removal of the unfavorable odor of vinasse so far. Thus, in this research, the volatile compounds present in vinasse produced by Razi yeast factory, which are among the wastes of this factory and have developed problems for its own surroundings and adjacent residential area, have been separated by GC technique after head space micro extraction. It is then detected and identified through mass spectrometry. In future studies, the fastest and least expensive way to convert these compounds to the more useful one as well as suitable solutions for removal of odor need to be presented, so that the local people would be free from the stinking odor emitted in the environment.
Materials and Methods
In this research, head space solid phase micro extraction (HS-SPME) technique was used and no other solvent or materials was utilized. The commercial fiber DVB/CAR/PDMS 50/30 μm was purchased from Supelco Co., USA. Vinasse was prepared from the ponds around Razi yeast production factory in 20-L containers and was mixed with each other and composite samples were prepared. The samples were transferred to laboratory and kept at a cool place until the analysis was done. In this research, DVB/CAR/PDMS commercial fiber with a thickness of 50/30 μm was employed as the absorbent of the volatile components of vinasse.
Preparation of DVB/CAR/PDMS fiber
To remove the contaminants absorbed on the fiber and prepare it for the first time, the fiber of interest was exposed to 200°C for 30 min at the injection port of the gas chromatography device. Thereafter, to record the control chromatogram, the fiber was exposed to the same temperature for 5 min at the same site and the chromatography program was run and the results were recorded. The chromatogram resulted at this stage might contain compounds such as commercial fiber adhesive, which was subtracted from the sample chromatogram when required.
Gas chromatography-mass spectrometry (GC/MS)
The gas chromatograph made by Agilent (7890A) equipped with mass spectrometer (5957C) was used. Separation was done on capillary column Hp5 ms with a length of 30 m, cross-sectional area of 250 mcm and stationary phase diameter of 0.25 μm. The auxiliary heater temperature was 275°C and the injection port temperature was 250°C. The helium with a purity of 99.999% and a flow rate of 1 mL/min was passed through the column. The column temperature program was as follows: first the oven temperature was kept at 40°C for 1 min, then it reached 300°C with a rate of 5°C/min and was kept at this temperature for 3 min. Data collection and device control was performed by Chemstation software 5.51. Library Wiley 7.1 and NIST were used for detection of the compounds.
The method of HS-SPME GC/MS
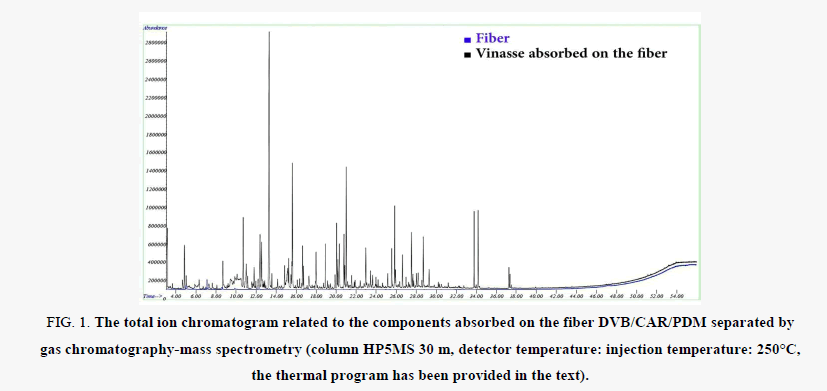
In a special 20-ml vial for analysis of gas vapors with GC which had been purchased from Agilent Company, 10 ml of liquid vinasse was poured. Next, the vial was immersed in the water bath of an ultrasonic device. The ultrasonic power was adjusted in a way that the vinasse solution was exposed to 60°C for 30 min. Thereafter, the fiber of interest, following pre-treatment, was placed on the top of the solution for 30 min, so that the volatile compounds present in the headspace would be absorbed by it. Eventually, the fiber was placed at the injection port of GC/MS for 2 min and the temperature program of the GC was run immediately and the data was recorded. The related chromatogram has been provided in Figure 1. To determine Kovat’s index, the mixture of samples containing aliphatic hydrocarbons with 8-32 carbons (C8-C32 TPH) were injected into GC device under similar conditions. Figure 1 represents the background spectrum by placing the finder before absorption of the volatile compounds of vinasse at the injection port and running the separation with a similar program.
Figure 1: The total ion chromatogram related to the components absorbed on the fiber DVB/CAR/PDM separated by gas chromatography-mass spectrometry (column HP5MS 30 m, detector temperature: injection temperature: 250°C, the thermal program has been provided in the text).
Results
The chromatogram associated with the volatile components absorbed on the fiber is shown in Figure 1. Table 1 provides the compounds which have been obtained through library search on the separated compounds. In this table, it has also been specified that some compounds have been confirmed through Kovat’s index with the cases reported in credible literature resources. As can be seen in Table 1, solid phase extraction with the fiber DVB/CAR/PDMS has detected 32 compounds present in the vapors of vinasse at 60°C, where 19 compounds had a frequency of over 2%. Out of the compounds, eight compounds, as provided in Table 2, generate odor, most of which are phenyl alcohol with a frequency of 26.52%. Around 42% of the compound separated by this fiber was among the odorous compounds (Table 2).
| Compound no. | RT (min) |
Kovats index (KI) |
Compound name | Normalized percent | Methods of identification |
|---|---|---|---|---|---|
| 1 | 3.173 | ------- | Hydrazine, 1,1-dimethyl- | 0.86 | a |
| 2 | 4.878 | 220.1361 | Furan, 2,3,5-trimethyl- | 2.49 | a |
| 3 | 8.700 | 860.1019 | Benzaldehyde, Phenylmethan | 1.34 | a |
| 4 | 10.726 | 1031.453 | dl-Limonene; | 4.02 | a, b, c, d |
| 5 | 11.029 | 1042.027 | Benzene methanol; Benzyl alcohol | 2.39 | a |
| 6 | 11.819 | 1068.285 | Ethanone, 1-(1H-pyrrol-2-yl)- | 1.06 | a |
| 7 | 12.402 | 1086.56 | Cyclotrisiloxane, hexamethyl- | 2.71 | a |
| 8 | 12.551 | 1091.093 | Phenol, 2-methoxy- | 2.02 | a |
| 9 | 13.318 | 1117.611 | Benzeneethanol; Phenethylalcohol | 26.52 | a, b, c, d |
| 0 | 14.845 | 1170.931 | Phenol, 4-ethyl-; p-Ethylphenol | 1.16 | a |
| 11 | 15.257 | 1184.378 | 1,2-Dihydropyridine, 1-(1-oxobutyl)- | 1.43 | a, c, d |
| 12 | 15.572 | 1194.417 | Phenol, 2-methoxy-4-methyl- | 1.04 | a |
| 13 | 15.624 | 1196.054 | Benzoic acid, 2-hydroxy-, | 6.49 | a |
| 14 | 16.648 | 1237.643 | Benzenepropanol | 2.26 | a |
| 15 | 16.728 | 1240.897 | Cyclotetrasiloxane, octamethyl- | 1.05 | a |
| 16 | 17.987 | 1290.159 | Phenol, 4-ethyl-2-methoxy- | 2.19 | a |
| 17 | 18.925 | 1324.669 | 2-Methoxy-4-vinylphenol $$ Phenol, | 2.31 | a, b |
| 18 | 20.093 | 1365.324 | Phenol, 2-methoxy-3-(2-propenyl)- | 3.08 | a |
| 19 | 20.207 | 1369.165 | 4-pentylbutan-4-olide | 1.43 | a |
| 20 | 20.344 | 1373.752 | Phenol, 2-methoxy-4-propyl- | 2.86 | a |
| 21 | 20.813 | 1389.224 | .beta.-Damascenone; 2-Buten-1-one | 2.66 | a |
| 22 | 20.899 | 1392.024 | Cyclohexanecarboxylic acid, 1-phenylDecanoic acid, ethyl ester | 1.04 | a |
| 23 | 21.042 | 1396.653 | Decanoic acid, ethyl ester | 5.32 | a, b |
| 24 | 22.994 | 1481.164 | Cyclodecane | 1.80 | a |
| 25 | 25.574 | 1584.182 | Megastigmatrienone | 1.94 | a, c, d |
| 26 | 25.860 | 1594.956 | Dodecanoic acid, ethyl ester | 3.32 | a, c, d |
| 27 | 26.644 | 1631.943 | Megastigmatrienone | 1.64 | a, c, d |
| 28 | 27.543 | 1674.929 | Phenol, 2,4-bis (1,1-dimethylethyl) | 2.93 | a |
| 29 | 28.721 | 1729.178 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl | 2.47 | a, c, d |
| 30 | 33.785 | 1975.829 | E-11-Hexadecenoic acid, ethyl ester | 3.57 | a, b |
| 31 | 34.180 | 1994.803 | Hexadecanoic acid, ethyl ester | 3.60 | a |
| 32 | 37.253 | 2165.388 | Linoleic acid ethyl ester | 0.98 | a, c |
a) MS Library search; b) Literature search; c) Phero base matching KI; d) Adams book [9]
Table 1. The chemical compounds detected in the head space of vinasse at 60°C by HS-SPME GC/MS using DVB/CAR/PDMS fiber.
| No. | Compound | Normalized percent |
|---|---|---|
| 1 | Limonene | 4.02 |
| 2 | Benzeneethanol | 26.52 |
| 3 | Phenyl 2-methoxy | 2.02 |
| 4 | Benzene propanol | 2.26 |
| 5 | Phenol 4-ethyl | 1.16 |
| 6 | beta-Damascenone | 2.66 |
| 7 | Benzyl alcohol | 2.39 |
| 8 | Decanoic acid, ethyl ester | 5.32 |
| Total odor % | 46.35 |
Table 2. The list of the odorous compounds detected through HS-SPME GC/MS method along with PDMS/CAR/DVB fiber in the head space of vinasse produced by Ahwaz Razi Yeast Company.
Discussion
The vinasse sample was exposed to 60°C in the GC vial. This temperature is the mean temperature that the lagoons around the Razi alcohol production factory tolerate in summer. Typically, when the city temperature reaches above 45°C and especially when wind blows, a stinking odor is emitted in the surrounding space from these lagoons. Therefore, the temperature of 60°C for 1 h (0.5 h to let vinasse vapors reach equilibrium with the vinasse solution and the second 0.5 h for exposure of fiber to vinasse vapors for maximum absorption) was applied to the vial and the compounds absorbed on the fiber were injected into the GC/MS device, based on the conditions mentioned above. At first, the separated compounds were listed through library search of the device. Then, by having the chromatogram of the sample and chromatogram of the aliphatic hydrocarbons, Kovat’s index was calculated for the compounds through eqn. 1.
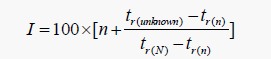
Lower number of carbon atoms and n denotes the number of carbon atoms in the alkane with a large number of carbon atoms and tr represents the retention time for each of the compounds. Comparing Kovat’s index with papers, Adam’s book and Pherobase database, the compounds were detected [6-14]. The detection method for each compound has been provided in Table 1
To determine the amount of separated compounds, area normalization method was used. This method relatively determines the level of volatile compounds of vinasse, which the present fiber has been able to absorb. In this method, the area of each of the peaks is divided by the sum of the entire area of the separated peaks and then multiplied by 100. The results have been provided as normalized percentage in Table 1 in front of each detected compound. Note that some of the compounds separated on the fiber (Table 1) have more than one isomer, some of which have been separated each other and can be observed in this table. Among these compounds is Megastigmatrienone. This compound has five isomers, which the major taste of tobacco is due to the presence of this compound. Besides, the compounds separated by this technique are more than the compounds detected by Lima et al. with dry powder of vinasse after separating it through Clevenger apparatus (17 compounds) [10]. In their study, phenyl ethyl alcohol (22.28%) was identified as the major compound. In this study, 15 other compounds were also identified, which probably degrade in the drying process.
The odorous compounds listed in Table 2 have different uses. Phenyl alcohol is a colorless organic liquid with the odor of flower and constitutes a major compound of plant essential oils. Therefore, it is used as a fragrant in perfumes and as a preservative in soaps. It has also antibacterial properties [15,16]. Alcohol benzyle is used as a solvent for inks, waxes, dyes, etc. This compound is also used as a raw material in soap making, perfume making and flavoring industries. At a concentration of 10%, it is used on wounds as a disinfectant and antimicrobial compound. It is also used in cosmetic and therapeutic materials. Its 5% solution has been confirmed by FDA for Lice treatment in children above six months of age as well as adults [17].
Limonene is a liquid colorless hydrocarbon which is a type of circular terpene. Its d-isomer has an extreme orange odor. In chemical synthesis, it acts as a raw material and used across different household detergents as a new compound. Limonene is widely used in cosmetic compounds. Due to the extreme odor of citrus compounds they are widely used in food industry and some medications. For instance, it can be used as flavoring for eliminating the bitter taste of alkaloids and as a fragrant in production of perfumes or aftershave lotions, as well as bathing products and other similar aromatic compounds. Its d-isomer acts better as a herbal insecticide and it is a good insecticide. In medicine, d-limonene has been used as anti-reflux and anti-heartburn [18]. In addition to health and medical uses, it is also considered as a biofuel since it has the potential to be burnt. Damascenone is another compound present in extracts. It belongs to a group of chemicals called rose ketones and beta-Damascenone is the aromatic compound present in rose flower. At even low concentrations, it has a strong odor and is used in production of perfume and aromatic materials [19].
Ethyl ester decanoic acid is a flavoring, as well as fatty acid ester which is produced from the reaction between capric acid and ethanol [20].
Conclusion
Therefore, considering the wide applications of the known odorous compounds, it is suggested that their separation be performed andutilized. Note that most of them are aromatic compounds, yet when their odor mixes with each other, it becomes annoying and unfavorable. The employed technique was fast and simple and can detect the volatile compounds present in vinasse with high accuracy without using any extraction solvent.
Acknowledgment
This study is derived from Ms. Palash’s dissertation and the authors would like to thank the student committee of the Office of research and technology development of Ahvaz Jundishapur University of medical sciences for their financial support (Gp95037).
References
- Elhami M, Jaffari S. Effect of vinasse as potassium fertilizer on chemical properties of soil. Campus of Agriculture and Natural Resources of Tehran University, 10th Iranian Soil Science Congress. 2009.
- Tejada M, Gonzalez J, García-Martínez A, et al. Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour Technol. 2008;99(11):4949-57.
- Baez-Smith C. Anaerobic digestion of vinasse for the production of methane in the sugar cane distillery. SPRI Conference on Sugar Processing, Loxahatchee, Florida, USA; 2006.
- Lalov IG, Krysteva MA, Phelouzat JL. Improvement of biogas production from vinasse via covalently immobilized methanogens. Bioresour Technol. 2001;79(1):83-5.
- Méndez-Acosta HO, Femat R, Campos-Delgado DU. Improving the performance on the chemical oxygen demand regulation in anaerobic digestion. Ind Eng Chem Res. 2004;43(1):95-104.
- Xu Y, Zhou X, Zhang D, et al. Headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometric (GC-MS) analysis of volatile profiles during the stir-frying process of malt. Anal Methods. 2016;8(7):1699-704.
- Zhao S, Zhang Y, Zhang X, et al. HS-SPME-GC MS analysis of trace volatile components in fermented grains from liquor production. Food Sci. 2013;34:118-24.
- Honggao Xu, Xiang Xu, Yidi Tao, et al. Optimization by response surface methodology of supercritical carbon dioxide extraction of flavour compounds from Chinese liquor vinasse. Flavour Fragr J. 2015;30(4):275-81.
- Adams RP. Identifications of essential oil components by gas chromatography mass spectrometry. 4th ed. Allured Publishing Corporation. 2007.
- Lima AVA, Barbosa MAS, Cunha LCS, et al. Volatile compounds obtained by the hydrodistillation of sugarcane vinasse, a residue from ethanol production. Revista Virtual de Química. 2017;9(2):764-73.
- Van Lancker F, Adams A, Delmulle B, et al. Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J Environ Monit. 2008;10(10):1127-33.
- Dayun Zhao, Jian Tang, Ding X. Analysis of volatile components during potherb mustard (Brassica juncea, Coss.) pickle fermentation using SPME-GC-MS. LWT-Food Science and Technology 2007;40(3):439-47.
- Márquez V, Martínez N, Guerra M, et al. Characterization of aroma-impact compounds in yerba mate (Ilex paraguariensis) using GC-olfactometry and GC-MS. Food Res Int. 2013;53(2):808-15.
- Moghimipour Z, Sourestani MM, Ansari NA, et al. The effect of foliar application of zinc on essential oil content and composition of holy basil (Ocimum sanctum) at first and second harvests. Journal of Essential Oil Bearing Plants. 2017;20(2):449-58.
- Fahlbusch KG, Hammerschmidt, Franz-Josef, et al. Flavors and fragrances. Ullmann's Encyclopedia of Industrial Chemistry. 2003.
- Lingappa B, Prasad M, Lingappa Y, et al. Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans. Science. 1969;163(3863):192-4.
- Bruhne F, Wright E. Benzyl alcohol. Ullmann's encyclopedia of industrial chemistry. 7 ed. Wiley. 2007; pp:7-8.
- Sun J. D-limonene: Safety and clinical applications. Altern Med Rev. 2007;12(3):259-64.
- http://www.leffingwell.com/rose.htm
- Killian E, Ough CS. Fermentation esters: Formation and retention as affected by fermentation temperature. Am J Enol Vitic. 1979;30(4):301-5.