Original Article
, Volume: 16( 1)Green Synthesis, Characterization and Antimicrobial Activity of Vanadium Nanoparticles using Leaf Extract of Moringa oleifera
- *Correspondence:
- Bognet O, Department of Chemistry, Nigerian Defence Academy, P. M. B. 2109, Kaduna, Nigeria, Tel: +2348030560543; E-mail: bognetzanan@gmail.com
Received: November 22, 2017; Accepted: December 26, 2017; Published: January 02, 2017
Citation: Aliyu AO, Garba S, Bognet O. Green Synthesis, Characterization and Antimicrobial Activity of Vanadium Nanoparticles using Leaf Extract of Moringa oleifera. Int J Chem Sci. 2017;16(1):231
Abstract
In this study, Vanadium nanoparticles (VNps) were synthesized using Moringa oleifera leaf extract as both reducing and stabilizing agent using green method of synthesis and characterized using Ultraviolet (UV) spectrophotometer, pH, Conductivity, Fourier Transform Infrared (FT-IR) spectroscopy, Scanning Electron Microscopy (SEM). The Antimicrobial activity of the synthesized nanoparticle was tested against Bacteria and fungi. The determination of pH at different time interval shows a gradual decrease as a function of time and stabilizes from 14-24th hour this confirm the stability of the nanoparticles synthesized. The FTIR spectrum reveal the functional groups responsible for the reduction and synthesis of the nanoparticles, this is obvious from the two spectra of biomass and Vanadium that the flavones and phenolic led to the bio-reduction. The SEM Analysis reveals disperse particles that are spherical in shape and a diameter of 100 nm which is due to the different capping agent that adsorb to the surface of the metal. The antimicrobial activity showed a minimal inhibitory effect on bacteria with zone of inhibition (ZOI) of 16 mm for Escherichia coli and 18 mm for Salmonella typhi, but no ZOI on fungi. It can be concluded that Vanadium nanoparticles synthesized using Moringa oleifera can be applied in medicine, as a curative agent.
Keywords
Vanadium nanoparticles; Moringa oleifera; Antimicrobial activity; Nanoparticles
Introduction
Nanoparticles
In the last decade, nanostructured vanadium compounds have attracted much interest due to their chemical and physical properties and their great potential for applications in catalysis, as sensors [1], in electrochromic and optical switching devices [2], as an electrode material for electrochemical capacitors and in windows for solar cells [3]. Nanotechnology is creating a growing sense of excitement in life sciences especially biomedical devices and biotechnology [4]. Nanoparticles are routinely defined as microscopic particles with sizes between 1 nm and 100 nm that show properties that are not found in bulk samples of the same material [5]. In this size range, the physical, chemical and biological properties of the nanoparticles changes in fundamental ways from the properties of both individual atoms/molecules and of the corresponding bulk materials. Nanoparticles can be made of materials of diverse chemical nature, the most common being metals, metal oxides, silicates, non-oxide ceramics, polymers, organics, carbon and biomolecules. Nanoparticles exist in several different morphologies such as spheres, cylinders, platelets, tubes etc. Generally, the nanoparticles are designed with surface modifications tailored to meet the needs of specific applications they are going to be used for. The enormous diversity of the nanoparticles arising from their wide chemical nature, shape and morphologies, the medium in which the particles are present, the state of dispersion of the particles and most importantly, the numerous possible surface modifications the nanoparticles can be subjected to make this an important active field of science now-a-days.
Properties of nanoparticles
A bulk material should have constant physical properties regardless of its size but at the nano scale, these are not often the case. They are effectively a bridge between bulk materials and atomic or molecular structure. Nanoparticles exhibit a number of special properties relative to bulk materials. Nanoparticles often display different chemical, physical, and biological characteristics, and thus behave differently, with respect to their bulk materials, even when the elemental or molecular composition is the same. Some of their properties can be extrapolated from the macro scale, whereas others change drastically below a certain size. Some characteristic of nanoparticles includes; possession of a high surface area to volume ratio, Possession of UV blocking properties, ability to conduct processes at lower temperature, lower melting temperature, ability to form suspension [6].
Classification of nanoparticles
Nanoparticles can be broadly grouped into two: namely organic and inorganic nanoparticles. Organic nanoparticles may include carbon nanoparticles (fullerenes) while some of the inorganic nanoparticles may include magnetic nanoparticles, noble metal nanoparticles (like gold and silver) and semiconductor nanoparticles (like titanium dioxide and zinc oxide) [7].
Nanotechnology has a wide range of applications in the fields of biology, medicine, optical, electrical and mechanical field.
Materials and Methods
Collection of plant material and preparation of extract
Fresh leaves of plant (Moringa oleifera) was collected from farms in Kudendan, Kaduna South LGA, Kaduna State, and was identified and authenticated at the Department of Biology, Kaduna State University, Nigeria with a voucher No. 7118, US Gallah. The collected leaves were washed with deionized water and chopped into pieces. A hot water extract of the leaf was prepared by taking 5 g of leaf in 100 mL of distilled water and boiled in an Erlenmeyer flask for 5 minutes. The extract colour was observed during experiment. The clear extract was obtained by filtration using Whatman filter paper. The extract was stored at 4?C for further use (it was used within one week). The filtrate acts as reducing and stabilizing agent for the synthesized Vanadium Nanoparticles [7,8].
Synthesis of vanadium nanoparticles
The aqueous solution of 1 mM Vanadium (II) Chloride was prepared to synthesize VNPs. 190 mL of aqueous solution of 1 mM VCl2 was slowly added to 10 mL of M. oleifera aqueous leaf extract while stirring, for reduction of V2+ into V0 [9,10]. The mixture was stirred vigorously on a magnetic stirrer for two hours till a colour change is observed. The mixture was centrifuged at 10,000 rpm for 20 minutes at room temperature. The supernatant was discarded, pellet re-dispersed in distilled water and lyophilized to dryness and stored at room temperature for future use [8].
UV-visible spectra analysis
UV-Visible spectrum of the reaction medium was used with model Spectrum lap 752 s to determine the reduction and stability of Vanadium nanoparticle.
Scanning electron microscopic analysis
SEM analysis of the VNP was performed with scanning electron microscope with model Phenom Prox. The particle was coated with a gold coating in order to have a good conductivity [8].
Determination of physico-chemical properties of vanadium nanoparticles
The pH was determined using pH meter, of model HI 2211 pH/ORP.
Fourier transmission infrared spectroscopy analysis
The FTIR spectrum of VNPs was performed using FTIR with model to identify the possible bio-molecules responsible for capping and efficient stabilization of metal nanoparticles synthesis using extract of M. oleifera. VNP pellet was washed 3 times with 20 mL of distilled water to get rid of all the free proteins/enzymes that were not capping the VNP prior to the FTIR measurement. The Sample was dried and analyse with FTIR machine in the diffuse reflectance mode using Attenuated Total Reflectance (ATR).
Antimicrobial activity
Pure bacteria (Salmonella typhi and Escherichia coli) and fungi (Candida albicans and Candida tropicalis (Yeast) species obtained from Microbiology Department, Kaduna State University, and were used. The antimicrobial activity of biosynthesized Vanadium nanoparticles was performed using disc diffusion method. A nutrient agar and potato dextrose agar medium plate were prepared, sterilized and solidified. After solidification, bacteria and fungi culture were swabbed on these plates. The sterile discs were dipped in VNPs solution (5 mg/mL) and placed in the nutrient agar plate and incubated at 37°C for 24 hrs. Zones of inhibition for control and test sample were measured [11].
Result and Discussion
Physicochemical properties
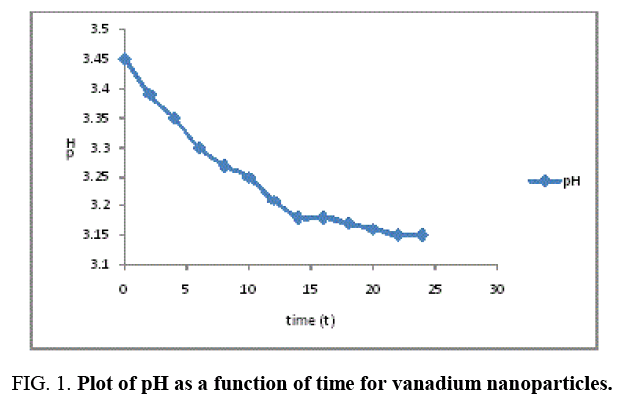
The result of the pH analysis shows that Vanadium nanoparticles synthesized using Moringa oleifera was analyzed at relative humidity of 75% and room temperature 27°C, before the synthesis, the pH of Vanadium solution and moringa leaf extract were 3.33 and 5.91 respectively indicating that the vanadium salt is more acidic compared to moringa leaf extract, and after the synthesis the pH of the Vanadium nanoparticles at different time interval shows a gradual decrease as a function of time and stabilizes at 14-24th hour as indicated in Figure 1, the mean pH value for the vanadium nanoparticles was 3.25 and was in the range of 3.15 to 3.45, this shows that the synthesized vanadium nanoparticles is acidic.
The Conductivity of the Vanadium nanoparticles synthesized using moringa leaf extract as a function of time shows a gradual increase indicating the presence of particles in the synthesize particle as shown in Figure 2. The mean conductivity value was 0.69 S/m and within the range of 0.013-0.096 S/m the high increase over time may be attributed to concentration of dissolved ions present in vanadium nanoparticles as Vanadium has distinctive properties such as good conductivity, catalytic and chemical stability.
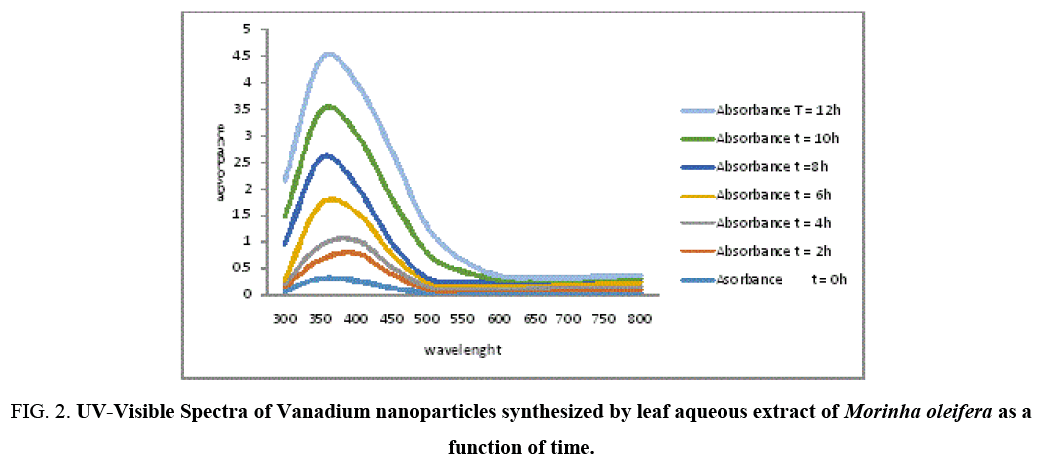
Figure 2: UV-Visible Spectra of Vanadium nanoparticles synthesized by leaf aqueous extract of Morinha oleifera as a function of time.
UV spectroscopy
Formation and stability of VNPs in deionized water is confirmed using UV-Vis spectrophotometer in a range of wavelength from 200 to 800 nm. As soon as, Moringa leaves extract was mixed in aqueous solution of vanadium ion complex, the reduction of V2+ ions to V0 was monitored by measuring UV–Vis spectrum of the reaction media at regular intervals. UV-Vis spectra were recorded as function of reaction time. The mixture showed a gradual change in color at room temperature with time from light yellowish green dark yellowish green and the color intensified after 12 hours (Figure 3), The color change is due to the Surface Plasmon Resonance phenomenon [12,13]. After 6 hours the Surface Plasmon Resonance of silver occurs at 360 nm and steadily increasing with the time of reaction without much change in the peak wavelength.
The appearances of dark yellowish color in the reaction vessels suggest the formation of Vanadium nanoparticles [8] and maximum absorption observed at 360 nm as time increases showed the formation of vanadium nanoparticles whose appearance and ration increases with time [14]. Researchers have reported that there are three different routes for the reduction of vanadium in plant extracts. The secondary metabolites (Flavonoids, Phenolics, Tarpenoids and Tannins) present in plant systems may be responsible for the reduction of Vanadium and synthesis of nanoparticles. The second biogenic route is the energy (or) electron released during glycolysis (photosynthesis) for conversion of NAD to NADH led to transformation of VCl2 to form nanoparticles [15,16] and another mechanism is releasing of an electron when formation of ascorbate radicals from ascorbate reduces the Vanadium ions [17].
FTIR spectrum of vanadium nanoparticles
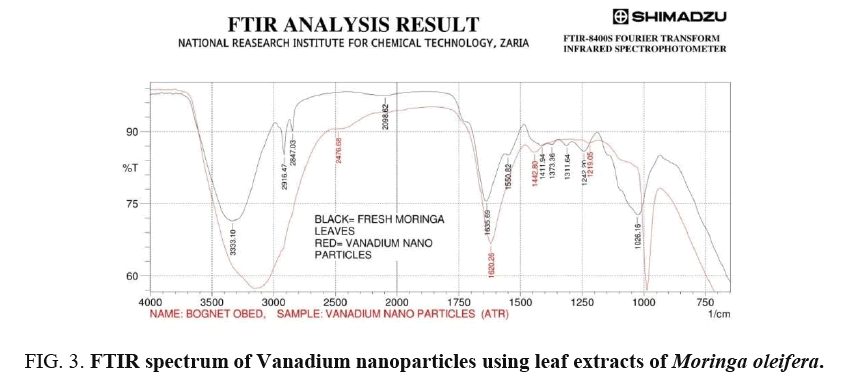
The analysis of IR spectra throws more light on biomolecules bearing different functionalities that are found attached to the surface of the synthesized nanoparticles. The FTIR measurement was carried out in the wave number range from 750-4000 cm-1 at room temperature as shown in Figure 4 for green synthesis of VNps and Moringa oleifera leaf extract respectively [18-20].
Broad peak in higher energy region at (3333.1-3155.65 cm-1) is due to stretching vibration of the O – H group. The peak at (1689.7-1620.26 cm-1) is due to amide that are characteristic of protein, indicating predominantly surface capping specie having C=O group that are responsible for stabilization. Peak at 1026.16-987.59 cm-1 and 1242.2-1219.05 cm-1 are due to C-O group of polyol Viz; Flavones terpenoids that are present in the biomass.
The overall observation proves the existence of some phenolics (OH) compounds, flavones, terpenoid and proteins that are bound to the surface of Vanadium nanoparticles. Changes observed in FTIR spectra of green synthesis of Vanadium nanoparticles after bio-reduction indicated the participation of terpenoid polyols and proteins having functional group of amides, alcohols and carboxylic acid in bio-reduction reaction. Terpenoid are poorly water-soluble and hence may not take part in the reduction of Vanadium ion. However, protein seemed to exhibit little importance in biosynthesis of nanoparticles. Therefore, water-soluble phenolics acid and flavonoid compounds are believed to play a major role in the bio reduction reaction of the synthesized nanoparticles.
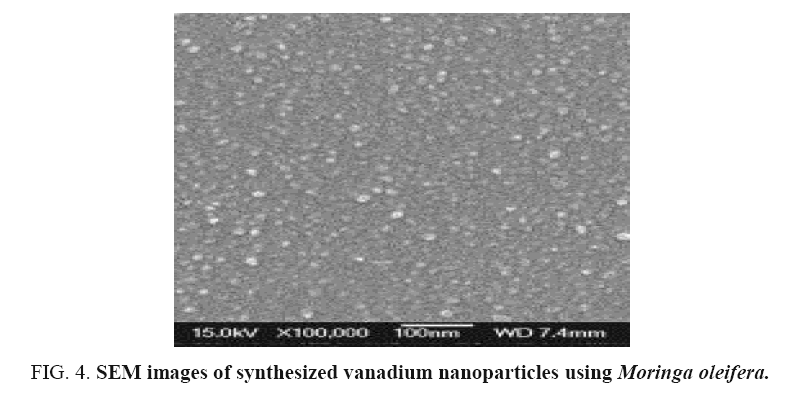
SEM analysis of vanadium nanoparticles
The particles were coated with a gold coating in order to have a good conductivity. They SEM image obtained showed biosynthesized VNPs are mostly Spherical in shape, this may be due to availability of different quantity and nature of capping agents present in the different leaf extracts which was supported by the shifts and difference in areas of the peaks obtained in the FTIR analysis. Thus, the green method is producing polydisperse nanoparticle of varying sizes.
Antimicrobial activity of vanadium nanoparticles
The Vanadium nanoparticles synthesized showed a minimal inhibition zone against bacteria strain (Escherichiacoli and Salmonella typhi), but no ZOI in fungi strain (Candia albican and Candida tropicalis). The ZOI of VNPs for Escherichia coli was 16mm and ZOI for Salmonella typhi was 18 mm less than that of the standard antibiotic Ciprofloxacin which was 40.50 mm and 39.00 mm respectively. This shows that vanadium nanoparticles show a minimum activity on bacteria strains (Table 1). The exact mechanism of the antibacterial effect of vanadium ions is partially understood.
| Organism | ZOI (mm) vnps | ZOI (mm) Standard drugs |
|---|---|---|
| Escherichia coli | 16.00 | 40.50 |
| Salmonella typhi | 18.00 | 39.00 |
| Candida albican | Nill | 40.00 |
| Candida tropicalis(Yeast) | Nill | 30.00 |
Table 1: Zone of inhibition of VNPs synthesized from Moringa oleifera leaf extract.
The chelate complexes deactivated various cellular enzymes which play vital role in various metabolic pathways of these microorganisms. This process, in turn, increases the lipophilic nature of the central metal atom, which favours its permeation more efficiently through the lipid layer of the microorganism [21,22] thus destroying them more aggressively.
The antibacterial activity is probably derived, through the electrostatic attraction between negatively-charged cell membrane of microorganism and positively-charged nanoparticles [23,24]. Also, accumulation of the VNPs in the pits results in the permeability of the cell membrane, causing cell death. However, [25] reported that the antimicrobial activity of metal nanoparticles on bacteria was dependent on the concentration of metal nanoparticles and was closely associated with the formation of pits in the cell wall of bacteria thus interfering with the ability of bacteria to continually form cell walls hence causing the cell wall to disintegrate.
Some other important factors such as nature of the metal ion, metal ion coordinating site, hydrophilicity and presence of co-ligands have considerable influence on the antibacterial activity [26]. Therefore, the antibacterial activity of the metal complexes cannot be ascribed to chelation alone but it’s an intricate blend of all of the above contributions.
Conclusion
The present study represents a clean, non-toxic as well as eco-friendly procedure for synthesizing VNPs. The capping around each particle provides regular chemical environment formed by the bio-organic compound present in the M. oleifera leaf broth, which may be chiefly responsible for the particles to become stabilized. This technique gives us a simple and efficient way for the synthesis of nanoparticles. The SEM observations, pH metre, Conductivity metre, UV Visible spectra, the FT-IR results and the antimicrobial analysis, confirmed the formation of Vanadium nanoparticles. The ability of Moringa oleifera to reduce Vanadium could be attributed to the phytochemical present in Moringa oleifera. The resultant material may be found a potential application in medicine with little modifications since there is a level of inhibition in bacteria (Escherichia coli and Salmonella typhi) but no inhibition in fungi organism.
From the technological point of view these obtained Vanadium nanoparticles have potential applications in the biomedical field and this simple procedure has several advantages such as cost-effectiveness, compatibility for medical and pharmaceutical applications as well as large scale commercial production.
References
- SubbaCV, ParkK, MhoS, et al. Simple preparation of V2O5. Nanostructures and their characterization. Bull Korean Chem Soc. 2008;29(10):2061-4.
- Al-ZoubiM, FaragHK,EndresH. Sol–gel synthesis of vanadium pentoxide nanoparticles in air- and water-stable ionic liquids. J Mat Sci. 2009;44:1363-73.
- Asim N, RadimanS, YarmoM, et al. Vanadium pentoxide: Synthesis and characterization of nanorod and nanoparticles V2O5 using CTAB micelle solution. MicropMesopor Mat.2009;120:397-401.
- PrabhuN, DivyaTR, YamunaG. Synthesis of silver phyto nanoparticles and their antibacterial efficacy. Digest JNanomatBiostruc. 2010;pp:185-9.
- Melanie A, JeromeR, JeanBY, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective nature nanotechnology.2009;4:634-41.
- KayserO, LemkeA, HernandezNT.The impact of nanobiotechnology on the development of new drug delivery systems. CurrPharmaBiotechnol. 2005;6(1):3-5.
- XuZP, YuAB.Inorganic nanoparticles as carriers for efficient cellular delivery.ChemEng Sci. 2006;61:1027-40.
- Ghosh N, Paul S, Basak P. Silver nanoparticles ofMoringaoleifera–Green synthesis, characterization and its antimicrobial efficacy. School of Bioscience and Engineering, Jadavpur University, India .
- JainD, DaimaHK, KachhwahaS, et al.Synthesis of plant-mediated silver nanoparticles using papapaya fruit extract and evaluation of antimicrobial activities.Digest JNanomat. Biostructure. 2009;4:55.
- SongJY,JangHK, KimBS. Biological synthesis of gold nanoparticles using magnolia kobus and Diopyros kaki Leaf Extracts.Process Biochem. 2009;pp:1133-8.
- http://www.microbiologyinfo.com/potato-dextrose-agar-pda-principle-uses-composition-procedure-and-colony-characteristics 2015.
- TamasaP. Synthesis and characterization of silver nanoparticles using leaf extract of Azadirachtaindica. 2013.
- Fawole MO, Oso BA.Laboratory Manual of Microbiology (Ibadan: Spectrum Books 2004) pp.8-35.
- XiaNY, HalasNJ. Shape control synthesis and surface plasmonicpropertis of metallic nanostructure. MRS Bulleting.2005;30:338.
- LabrenzM, DruschelGK, ThomsenET, et al. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science.2000;1:744-7.
- RothY, BaiJ, LaufRJ, et al. Microbial synthesis of metal-substitutedmagnetites. Solid State Commun. 2001;11:529-34.
- AhmadN, SharmaS, SinghVN, et al. Biosynthesis of silver nanoparticles fromDesmodiumtrifolium: A novel approachtowards weed utilization.Biotechnol Res Int. 2011;pp:1-8.
- Mukherjee P, Roy M, Mandal BP, et al.Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology. 2008;19(7):075103.
- NatalyT, MarcosN, AngelaBS, et al. Green synthesis of nanosized vanadium pentoxide using Saccharomyces cerevisiae as bio template.Rec Res Development. Mat Sci.2013;10:89-102.
- AnamikaM, SanjuktaC, PrashantM, et al. Evidence based green synthesis of nanoparticles. J AdvMat Lett.2013;3(6):519-25.
- Whitfield TR, Wang X, Liu L, et al. Metal-organic frameworks based on iron oxide octahedral chains connected by benzenedicarboxylatedianions. Solid State Sci. 2005;7(9):1096-103.
- HamoudaT, MycA, DonovanB.A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi.Microbiol Res. 2001;156(1):1-7.
- Dibrov P, Dzioba J, Gosink KK, et al. Chemi-osmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrobial Agents and Chemotherapy. 2002;46(8):2668-70.
- SondiI, Salopek-SondiBJ.Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. Colloid Interface Sci. 2004;275:177.
- LevingsonW, MikeleusP, JacksonJ, et al. Inorganic and nutritional aspects of cancer, GN Schranzer. Plenum Press, New York. 1978.
- ChohanZH, HassanMU, KhanKM.In-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide-derived Schiff bases and their metal complexes. J EnzInhib Med Chem. 2005;20:183-8.