Original Article
, Volume: 15( 2)Factors Affecting Acute Toxicity Dose of Lead Nitrate in Certain Indian Air-Breathing Fishes
- *Correspondence:
- Pandit DN , Department of Zoology, Veer Kunwar Singh University, Arrah, Bhojpur, India, Tel: +91-9454211574; E-mail: panditdina@gmail.com
Received: May 02, 2017; Accepted: June 05, 2017; Published: June 14, 2017
Citation: Pandit DN, Kumari R, Kumari V. Factors Affecting Acute Toxicity Dose of Lead Nitrate in Certain Indian Air-Breathing Fishes. Int J Chem Sci. 2017;15(2):144.
Abstract
The present work was conducted to determine an ideal median lethal concentration of lead nitrate in two air-breathing fishes namely Channa punctatus (Bloch) and Clarias batrachus (Linn.) using following methods and also influence of different factors to get at a logical conclusion. (1) Graphical method: The LC50 value was 12.02 and 165.0 mg/L for Channa punctatus while 350.0 and 380.0 mg/L for Clarias batrachus. (2) Probit Graphical method: The LC50 value was 11.48 and 177.8 mg/L for Channa punctatus while 315.0 and 346.7 mg/L for Clarias batrachus. (3) Regression method of Finney (1971): From this method LC50 value was calculated to be 11.75 and 208.0 mg/L for Channa punctatus while 320.0 and 348.0 mg/L for Clarias batrachus. (4) Behrens and Karber method: By this method, 96 h-LC50 was found 12.75 and 167.5 mg/L for Channa punctatus while 375.0 and 425.0 mg/L for Clarias batrachus. From these values, the average median lethal concentration of 96 h-LC50 value was calculated to be 12.0 mg/L for Channa punctatus (body weight: 50-68 g) and 180.0 mg/L for Channa punctatus (body weight: 55-75 g). Similarly, 340.0 mg/L for Clarias batrachus (body weight: 85-110 g) and 375 mg/L for Clarias batrachus (body weight: 125-150 g). In this paper, factors such as intra-specific variation, physico-chemical features, spatial variation and methodological variation affecting the values of LC50 has also been studied.
Keywords
Channa punctatus; Clarias batrachus; Lead nitrate; Acute toxicity
Introduction
Aquatic life is strongly affected by physico-chemical properties of a water bodies. It has been reported that heavy metals had a negative impact on different physico-chemical parameters and caused various changes in animals. The heavy metal concentration in the body of fish depends upon feeding habits, trophic status, food availability, physico-chemical properties of water, metabolic rate of animal and toxicity of heavy metals [1]. Lead is a non-essential and non-beneficial element. It had added a problem of health hazard to human and experimental mammals.
It has also received the attention over the past few years as potentially important aquatic pollutant. Acute toxicity describes the adverse effect of a heavy metal/other chemical on an animal resulting from single exposure. Acute toxicity tests are rapid procedures used for measuring the concentration of heavy metal, expressed as the median lethal concentration (LC50), which will affect the animal. It is the concentration of a substance in the solvent which kills 50% of the test batch of the fish or the other living organism within a stated continuous period of exposure.
Median lethal concentration of lead in various air-breathing fishes is reported [2-4]. However, effect of intra-specific variation, physico-chemical features, spatial variation and methodological variation on the value(s) of median lethal concentration in air-breathing fishes is lacking. Therefore, present work was conducted to study such factors on median lethal concentration of lead nitrate in two commercially important Indian air-breathing fishes namely, snake headed murrel, Channa punctatus (Bloch) and Indian catfish, Clarias batrachus (Linn.) to enhance reliability and to standardize the value.
Materials and Methods
In the present investigation, Channa punctatus (Body weight: 50-75 g) and Clarias batrachus (Body weight: 85-150 g) were collected from local fishermen of Arrah and adjacent localities during 2013-2015. The fishes were brought to the laboratory in polythene bags containing the pond water. The fishes were disinfected by washing properly with dilute KMnO4 and then transferred to many large aquaria (90 × 60 × 45 cm). The fishes were fed with pieces of goat’s liver and fish food available in the local market.
Fishes of each aquarium were thoroughly examined and unhealthy and injured specimens were rejected. To keep the aquaria free from fungal growth antifungal chemicals were used. The temperature of water of each aquarium was maintained at room temperate throughout the period of investigation and physico-chemical features of experimental water was determined. The experiments were conducted every time in between 11 AM to 1 PM.
The toxicity test of the lead nitrate [Pb(NO3)2] was conducted by employing the static bioassay method and recommendation for the toxicity test experiments [5,6]. Analytical Reagent grade chemicals (Merck India Co. Ltd) were used during experiments. Five different concentrations of lead nitrate were taken in separate aquarium. In each concentration, twenty fishes from the acclimatized fish stock were kept. The first set of experiment was conducted for 24 h, second for 48 h, third for 72 h and fourth for 96 h respectively. The experiments were monitored round the clock and numbers of fishes died during the experiments in each concentration of each set of experiment was recorded. The fishes were considered dead when they gave on response to touch with a glass rod. The whole sets of experiments were repeated thrice to get an average result and to minimize any error due to unavoidable reasons.
With the help of the records of dead fishes, 96 h-LC50 doses were determined by following methods:
(1) Graphical method taking percent mortality
(2) Graphical method taking log and probit mortality
(3) Regression method [7] and
(4) Behrens and Karber method
Statistical analysis was performed with the use of Graph Pad Prism 5.
Results
The physico-chemical features of experimental water have been given in Table 1. The data on determination of LC50 for different durations of lead nitrate on Channa punctatus (Bloch) and Clarias batrachus (Linn.) have been summarized in Tables 2 to 6. The lethal range was determined to sequential concentrations from 0.1, 0.01, 10.0, 100.0 to 1000.0 mg/L of lead nitrate [8]. 20 specimens of fishes were released separately in a fish aquarium containing 20 liters of water and sequential concentrations of lead nitrate were added. The mortalities were recorded at 24, 48, 72 and 96 hours of commencement of the experiment and dead fishes were removed immediately.
| Physico-Chemical Parameters | Values | |||
|---|---|---|---|---|
| Channapunctatus (2013) |
Channapunctatus (2015) |
Clariasbatrachus (2013) |
Clariasbatrachus (2014) |
|
| Water temperature (°C) | 25.0 ± 1.0 | 27.0 ± 1.0 | 25.0 ± 1.0 | 26.0 ± 1.0 |
| pH | 7.02 ± 0.5 | 7.41 ± 0.4 | 7.02 ± 0.5 | 7.08 ± 0.1 |
| Dissolved oxygen (mg/L) | 6.8 ± 0.4 | 6.1 ± 0.8 | 6.8 ± 0.4 | 6.5 ± 0.6 |
| Free Carbon dioxide (mg/L) | - | - | - | - |
| Chloride (mg/L) | 13.5 ± 2.8 | 12.95 ± 2.6 | 13.5 ± 2.8 | 13.8± 2.7 |
| Hardness (mg/L) | 107.2 ± 8.5 | 113.8 ± 10.4 | 107.2 ± 8.5 | 110.7 ± 5.8 |
| Total alkalinity (mg/L) | 113.5 ± 4.5 | 118.3 ± 8.4 | 113.5 ± 4.5 | 112.6 ± 5.2 |
Table 1: Physico-chemical parameters of experimental water.
| Group | Dose of lead nitrate (mg/L) | Difference between two consecutive dose (A) | No. of fish exposed | Mortality | Overall mortality at 96 h | Mean mortality between two consecutive dose (B) | AxB | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 72h | 96h | |||||||
| 1 2 3 4 5 6 |
0 5 10 15 20 25 |
0 5 5 5 5 5 |
20 20 20 20 20 20 |
0 0 0 0 3 5 |
0 3 6 7 10 12 |
0 4 7 9 13 17 |
0 5 8 12 16 20 |
0 5 8 12 16 20 |
0 2.5 6.5 10.0 14.0 18.0 |
0 12.5 32.5 50.0 70.0 90.0 |
| - | - | - | - | - | - | - | 255.0 | |||
96 h LC50=LC100-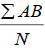 , = 25 ?
, = 25 ?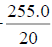 , =25 ? 12.75, =12.75 mg/L
, =25 ? 12.75, =12.75 mg/L
Table 2: Behren-Karber method for determination of 96 h-LC50 dose of lead nitrate in Channa punctatus.
| Group | Dose of lead nitrate (mg/L) | Difference between two consecutive dose (A) | No. of fish exposed | Mortality | Overall mortality at 96h | Mean mortality between two consecutive dose (B) | AxB | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 72h | 96h | |||||||
| 1 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 125 | 25 | 20 | 0 | 3 | 5 | 3 | 3 | 1.5 | 37.5 |
| 3 | 150 | 25 | 20 | 0 | 6 | 8 | 5 | 5 | 4.0 | 100.0 |
| 4 | 175 | 25 | 20 | 0 | 7 | 10 | 8 | 8 | 6.5 | 162.5 |
| 5 | 200 | 25 | 20 | 3 | 11 | 17 | 20 | 20 | 14.0 | 350.0 |
| -- | -- | -- | -- | -- | -- | 650.0 | ||||
96 h LC50=LC100 -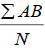 , =200?
, =200? 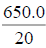 , =200-32.5, =167.5 mg/L
, =200-32.5, =167.5 mg/L
Table 3: Behren-Karber method for determination of 96 h-LC50 dose of lead nitrate in Channa punctatus.
| Group | Dose of lead nitrate (mg/L) | Difference between two consecutive dose (A) | No. of fish exposed | Mortality | Overall mortality at 96 h | Mean mortality between two consecutive dose (B) | AxB | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |||||||
| 1 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 250 | 250 | 20 | 4 | 6 | 8 | 8 | 8 | 4 | 1000 |
| 3 | 500 | 250 | 20 | 12 | 12 | 14 | 14 | 14 | 11 | 2750 |
| 4 | 750 | 250 | 20 | 16 | 18 | 18 | 18 | 18 | 16 | 4000 |
| 5 | 1000 | 250 | 20 | 16 | 20 | 20 | 20 | 20 | 19 | 4750 |
| -- | -- | -- | -- | -- | -- | -- | 12500 | |||
96 h LC50=LC1000-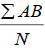 ,=1000-
,=1000-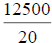 , =1000-625, =375.0 mg/L
, =1000-625, =375.0 mg/L
Table 4: Behren-Karber method for determination of 96 h-LC50 dose of lead nitrate in Clarias batrachus.
| Group | Dose of lead nitrate (mg/L) | Difference between two consecutive dose (A) | No. of fish exposed | Mortality | Overall mortality at 96h | Mean mortality between two consecutive dose (B) | AxB | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 72h | 96h | |||||||
| 1 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 250 | 250 | 20 | 0 | 3 | 5 | 8 | 8 | 4 | 1000 |
| 3 | 500 | 250 | 20 | 0 | 6 | 8 | 12 | 12 | 10 | 2500 |
| 4 | 750 | 250 | 20 | 0 | 7 | 10 | 16 | 16 | 14 | 3500 |
| 5 | 1000 | 250 | 20 | 3 | 11 | 17 |
20 |
20 | 18 | 4500 |
| -- | -- | -- | -- | -- | -- | -- | 11500 | |||
96 h LC50=LC100-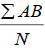 , = 1000 -
, = 1000 - 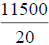 , =1000-575, =425.0 mg/L
, =1000-575, =425.0 mg/L
Table 5: Behren-Karber method for determination of 96 h-LC50 dose of lead nitrate in Clariasbatrachus.
| Name of fish | Body weight (g) | Water temperature (°C) | Year | Percentgraphicalmethod | Log-graphical method | Finney (1971) regression method | Behrens and Karbermethod | Average |
|---|---|---|---|---|---|---|---|---|
| Channapunctatus | 50-68g | 25.0 ± 1.0°C | 2013 | 12.02 mg/L | 11.48 mg/L | 11.75 mg/L | 12.75mg/L | 12.0 mg/L |
| Channapunctatus | 55-75g | 27.0 ± 1.0°C | 2015 | 165.0 mg/L | 177.8 mg/L | 208.0 mg/L | 167.5mg/L | 180.0 mg/L |
| Clariasbatrachus | 85-110g | 25.0 ± 1.0°C | 2013 | 350.0 mg/L | 315.0 mg/L | 320.0 mg/L | 375.0mg/L | 340.0 mg/L |
| Clariasbatrachus | 125-150g | 26.5 ± 1.0°C | 2014 | 380.0 mg/L | 346.7 mg/L | 348.0 mg/L | 425.0mg/L | 375.0 mg/L |
Table 6: Summary of 96 h-LC50 dose of lead nitrate determined by different methods in Channapunctatus and Clariasbatrachus.
(1) Graphical method taking percent mortality: The LC50 value of lead nitrate was found 12.02 and 165.0 mg/L for Channa punctatus while 350.0 and 380.0 mg/L for Clarias batrachus.
(2) Graphical method taking log and probit mortality: From this method, the LC50 value of lead nitrate was found 11.48 and 177.8 mg/L for Channa punctatus while 315.0 and 346.7 mg/L for Clarias batrachus.
(3) Regression method: From this method LC50 value of lead nitrate was calculated to be 11.75 and 208.0 mg/L for Channa punctatus while 320.0 and 348.0 mg/L for Clarias batrachus [7].
(4) Behrens and Karber method: In this method, a difference between two consecutive doses was determined and arithmetic mean of mortality caused by two consecutive doses was also calculated. Then product of difference between two consecutive doses and arithmetic mean of mortality caused by two consecutive doses was calculated. Finally, 96 h-LC50 value of lead nitrate was found 12.75 and 167.5 mg/L for Channa punctatus while 375.0 and 425.0 mg/L for Clarias batrachus. From these values, the average median lethal concentration of 96 h-LC50 of lead nitrate was calculated to be:
(a) 12.02+11.48+11.75+12.75=12.0 mg/L for Channa punctatus (body weight: 50-68 g) during 2013 at 25.0 ± 1.0°C.
(b) 165.0+177.8+208.0+167.5=180.0 mg/L for Channa punctatus (body weight: 55-75 g) during 2015 at 27.0 ± 1.0°C.
(c) 350.0+315.0+320.0+375.0=340.0 mg/L for Clarias batrachus (body weight: 85-110 g) during 2013 at 25.0 ± 1.0°C.
(d) 380.0+346.7+348.0+425.0=374.925 or 375 mg/L for Clarias batrachus (body weight: 125-150 g) during 2014 at 26.0 ± 1.0°C.
Discussion
Studies on the median lethal concentrations usually provide measure of relative toxicity of different heavy metals and also the sensitivity of fish species. Fishes are generally used in the evaluation of aquatic system quality and some of their physiological changes can be considered as biological markers of environmental pollution [9].
Lead toxicity is mainly a function of water hardness and nature of species along with age of fishes. Increased water hardness reduces lead toxicity due to a significant inorganic complexion process that controls lead availability to fish [10,11]. LC50 values differ from species to species for the same toxicant due to the mode of action and responses of the animals. The LC50 has been modified several times since its development in the 1920s depending on the type of animal, nature of substance, environmental factor, etc., There are different methods used in the determination of LC50 dose [7,12-15].
In this work, the average 96 h-LC50 dose of lead nitrate was calculated to be 12.0 mg/L and 180.0 mg/L during 2013 and 2015 respectively for Channa puncattus (Table 5). A dose of 96 h-LC50 was calculated 11.6 mg/L for lead nitrate in Channa punctatus [3]. In another work, this dose was reported to be 600.00 mg/L in Channa punctatus (body weight 50-90 g) [4].
However, all these works are in the similar lines. But, one of the works showed more than three times increase in LC50 dose of lead nitrate in Channa punctatus in comparison to the present work [4].
The molecular mass of lead nitrate is 331.2 g/mol. Therefore, 331.2 mg by part of lead nitrate contains 207.19 mg of lead or, 1 mg by part of lead nitrate will contain 207.19/321.2=0.645 mg of lead. It means that at least 50% of Channa punctatus can survive in a water body having 12.0 × 0.645=7.74 mg/L to 600.0 × 0.645=387.0 mg/L of lead in an inorganic or organic compound. The difference also depends on the phenotypic variations of fish. The statement is supported by a work in which they studied phenotypic variations in Channa punctatus and reported its three variants in India [16].
On the other hand, the average 96 h-LC50 dose of lead nitrate was calculated to be 340.0 and 375 mg/L during 2013 and 2014 respectively for Clarias batrachus (Table 5). During an experiment, it was calculated 96 h-LC50 value to be 378 mg/L in Clarias batrachus (body weight 80-100 g) [2]. In another work, it was also reported a 96 h-LC50 of lead nitrate as 300.45 mg/L in Clarias batrachus [17]. On the other hand, some other workers determined 48 h-LC50 of 500 mg/L lead as lead acetate in Clarias batrachus. It showed more than 1.25 times increase in LC50 dose of lead nitrate in Clarias batrachus [18]. Therefore, at least 50% of Clarias batrachus can survive in a water body having 300.45 × 0.645=194.0 mg/L to 500.0 × 0.645=323.0 mg/L of lead in an inorganic or organic compound.
The present work as well as earlier reports indicates some sort of spatial increment in LC50 dose of lead nitrate for the same fish of similar body weights. The increment may be due to increase in ambient temperature and adaptations shown by fishes. Further, the pattern of difference in LC50 dose of lead nitrate was related with the difference in the body weight of fishes. In this experiment, body weight ranged from 50-75 g and 85-150 g in Channa punctatus and Clarias batrachus respectively indicating a wider range in Clarias batrachus. It shows more active nature of Channa puncataus in comparison to Clarias batrachus. The active nature of Channa puncataus (b=0.802) and Clarias batrachus (b=0.763) was confirmed with their corresponding slope value of total oxygen uptake [19,20].
The values of various physico-chemical parameters of experimental water were more or less similar during this experiment. Therefore, physico-chemical parameters not seem to be major factors that influence LC50 dose of these fishes in this experiment. As far as determination of LC50 dose by different methods are concerned, they definitely affect the value of LC50 dose. Two-way ANOVA inferred that use of different methods determine has highly significant effect (p<0.001, F=186.2) on the value of LC50 dose. Therefore, it seems that selection of appropriate method to determine LC50 is one of the most important aspects in toxicological studies.
Conclusion
Therefore, it may be concluded that the variations in doses may be due to the differences in the age, body weight, nutrient supply and habitat as well as in the abiotic conditions. The values are different and dependent on the conditions under which tests were performed. It also infers that the tolerance limit for a toxicant by a fish is increasing gradually due to increase in resistance power.
References
- Yilmaz A, Turan C, Toker T. Uptake and distribution of hexavalent Cr in tissues (gill, skin and muscle) of a freshwater fish, tilapia, Oreochromisaureus. JEnviron ChemEcotoxicol. 2010;2:28-33.
- Nehar S, Kumar C, Mishra K, et al.Lead induced alternation in Clariasbatrachus.The Bioscan. 2010;6:337-41.
- Chaudhary J, JhaAM.Genotoxic Testing of Lead Nitrate In Air-Breathing Teleost Channapunctatus(Bloch). Internat J Plant Animal Environm Sci. 2012;2:229-34.
- Dutta B, Sarma SR, Parag D. Lead nitrate toxicity on haematological changes in a live fish species Channapunctatus(Bloch). Internat J Fisheries Aquat Stud.2015;3:196-98.
- Doudoroff P, Anderson BG, Burdick GE, et al.Bioassay methods for the evaluation of acute toxicity of industrial wastes to fish. Sew IndWas. 1951;23:1380-97.
- APHA. Standard Methods for the examinations of water and waste water. 17th ed. American Public Health Association, Washington 1989.
- Finney DJ. Profit analysis, 3rded. Cambridge University, Press. London, New York 1971.
- Reish DL, Oshida PI. Manual of methods in aquatic environment research part 10 short term static bioassay FAO fisheries technical report 247. FAO, Rome. 1987;pp:1-62.
- Rout PC, Naik BN. Tracing out correlation between blood lead and haematologicalparamteters in Clariasbatrachus during experimental plumbism. IJSRP. 2013;3:325-40.
- Demayo A, Taylor MC, Taylor KW, et al. Toxic effects of lead and lead compounds on human health, aquatic life, wildlife plants and livestock. Cri RewEnv Ctr. 1981;12:257-305.
- Hodson PV, Whittle DM, Wrong PTS, et al.Lead contamination of the great lakes and its potential effects on aquatic biota. Toxic Contaminants in Great Lake. John Willey and Sons 1984.
- Karber G. BeitrazurkovecktivenBehandlungpharmakologischerReihenversucbe. Arch Exp Path U Pharmakolol. 1931;162:480-83.
- Reed LJ, Muench H. A simple method for estimating fifty per cent end points. Am J Hyg. 1938;27:493-97.
- Miller LC, Tainter ML. Estimation of LD50 or ED50 values and their errors using Log-probit graph paper. ProcSocExptBiol Med. 1944;57:261-64.
- Litchfield JT, Wilcoxon FA.A simplified method of evaluating dose-effect experiments. J PharmacolExpTher. 1949;96:99-113.
- Kashyap A,Awasthi M,Serajuddin M.Phenotypic variation in freshwater murrel,Channapunctatus(Bloch, 1793) from Northern and Eastern Regions of India using Truss analysis. Internat J Zool. 2016.
- Khan, AH, Sikdar-Bar M, Kamlesh B, et al. Lead nitrate induced histopathological changes in the gills of the African, Clariasbartachus. J ApplSci Res. 2011;7:1081-88.
- Rout PC, Naaz A, Sahoo P. Testing lethal concentration of lead acetate on Clariasbatrachus. Linn Asi Res. 2013;2:76-82.
- Ghosh TK, Kunwar GK, Munshi JSD. Diurnal variation in the bimodal oxygen uptake in air-breathing catfish, Clariasbatrachus. Japan J Ichthyol. 1990;37:56.
- Patra AK, Munshi JSD, Hughes GM.Oxygen consumption of the freshwater Indian siluroid fish, Clariasbatrachus in relation to body size and seasons. Proc Ian Nat. Sci Acad. 1983;49:566.
