Original Article
, Volume: 16( 1)Experimental Study of the Elimination of a Textile Dye by the Adsorption Phenomenon on Beech Wood Sawdust
- *Correspondence:
- Adib M, Laboratory of Analytical Chemistry and Physical Chemistry of Materials, Faculty of Sciences Ben M'sik, Hassan II University of Casablanca, Morocco, Tel: +212658978020; E-mail: meriem.adib@gmail.com
Atmani R, Laboratory of Analytical Chemistry and Physical Chemistry of Materials, Faculty of Sciences Ben M'sik, Hassan II University of Casablanca, Morocco, Tel: +212-650030208; E-mail: atmanirachid12@gmail.com
Received Date: December 13, 2017; Accepted Date: January 08, 2017; Published Date: January 12, 2018
Citation: Adib M, El Kouali M, Talbi M, et al. Experimental Study of The Elimination of a Textile Dye by the Adsorption Phenomenon on Beech Wood Sawdust. Int J Chem Sci. 2018;16(1):239
Abstract
The purpose of this study is to use the beech sawdust that is very abundant for the treatment of methylene blue and to identify the effect of various physico-chemical factors of the medium that influence the ability of adsorption such as: Initial concentration, particle size, adsorbent mass and stirring speed. Experimental results show that the adsorption of methylene blue dye on beech sawdust depends of particle size and initial concentration of dye.
Keywords
Adsorption; Methylene blue; Dye; Wood; Sawdust
Introduction
The industrial development generates pollutants which affect directly the balance of the ecosystems following the degradation of the quality of the various environmental fields, in particular that of the waters. Consequently, the depollution of waters represents an essential purpose referred by many researchers. The intense application of synthetic dyes in these various industries generates polluting substances which require a specific treatment. There are several methods of water treatment whose purpose is to eliminate pollutants; the adsorption remains the most wide-spread technic [1]. The activities of the national scientific research in the environment field are directed to the development of technics of dyes elimination by adsorption on new less expensive natural materials due of their abundance as waste easily available in Morocco. The application of the diverse waste as adsorbents constitutes a very interesting means in the elimination of pollutants in waters and contributes thus the reduction of the environmental problems. The purpose of this study is to use the beech wood sawdust, obtained from beech as biosorbent in order to eliminate the cationic dye of the textile effluents [2-4]. The effects of various factors which characterize relatively the dye and the support of adsorption had been examined.
Materials and Methods
Preparation of adsorbent material
The sawdust obtained from the tree of the beech of a size granular corresponds to 160 μm was crushed and sieved in the laboratory for establish the various sizes granular suited which corresponds to the following diameters:
• d60 (the size of the sieve through which 60% of the sawdust crosses the sieve).
• d80 (the size of the sieve through which 80% of the sawdust crosses the sieve).
• d100 (the size of the sieve through which 100% of the sawdust crosses the sieve).
This sawdust was put in suspension in some distilled water and maintained under constant stirring for 3 hours so as to free the impurities. Then the suspension was filtered and dried in 105°C for 24 hours.
Dye
The chemical dye selected for this study is the methylene blue (BM) is a basic dye “organic cationic” belonging to the family of thiazine dyes, (Tetramethyl thionine chloride or methylthioninium chloride), which has the property to color durably the support to which it’s applied. It’s soluble in the water and more slightly in the alcohol [5].
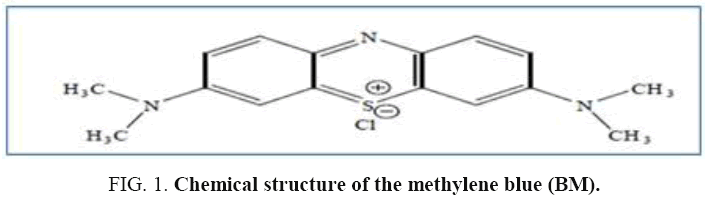
The chemical formula of the (BM) is (C16H18N3S, × H2O) where × represent the number of water molecules of crystallization (between 3 and 5); the Figure 1 shows the molecular structure of the methylene blue (BM).
The initial solution of 100 mg/l is prepared by dissolution of 100 mg of the dye in one liter of the distilled water. The solutions colored of various concentrations used in this study had been prepared by the process of dilution.
Absorption experiments
All the experiments of adsorption had been realized in Erlenmeyer of 250 ml batch process by pouring 100 ml of the solution prepared at an ambient temperature (22 ± 2°C). By putting in touch a mass (m) of particle size clearly defined with 100 ml of an aqueous solution of dye of concentration (10, 15 and 20 ppm) during a fixed time interval [6]. The suspensions obtained had been maintained under a constant stirring during the time required in order to reach the balance of adsorption. Adsorption time at equilibrium for the various concentrations of dye had been determined in preliminary kinetic tests. The measures and the spectral evolution of the optical density of the solutions of methylene blue in various time of reaction had been followed by UV/visible spectrometry (mini-Shimadzu UV-on 1240); Absorbance in 664 nm was registered so as to determine the remaining color in the solution (Cr), by using the Beer-Lambert law.
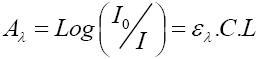 (1)
(1)
With:
- A: Absorbance;
- ελ: Specific extinction coefficient of the solution;
- L: Thickness of the optical cell;
- C: The concentration of the solution.
The adsorbed quantity is determined by the following relation:
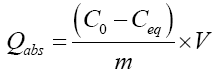 (2)
(2)
- Qads: Adsorbed quantity (mg/g);
- C0: Initial concentration (mg/L);
- Ceq: Concentration at equilibrium (mg/L);
- m: Adsorbent mass (m);
- V: Volume of the solution (L).
The yield so as to eliminate from the dye (BM) either the percentage of discoloration had been calculated by using the following formula:
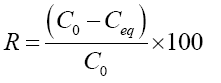 (3)
(3)
For our study, concentrations solutions clearly determined had been prepared and calibrated by the technic of precipitated analysis.
Results and Discussion
Optimization of the conditions of adsorption of the methylene blue on the sawdust of the beech wood
In order to optimize the conditions of adsorption of the methylene blue on the beech wood sawdust, we have studied the effect of a series of factors susceptible to act in the process of this phenomenon [7].
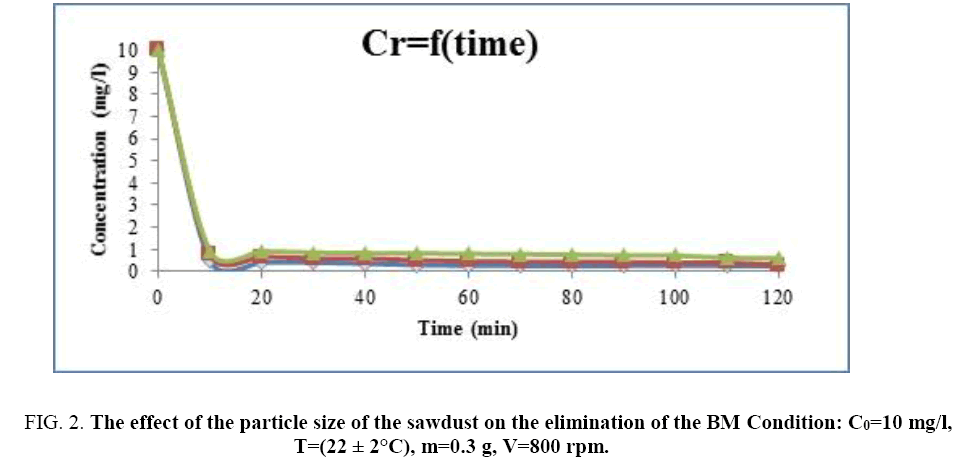
Effect of the particles size: Three types of particle size of the sawdust had been used to study their effects on the adsorption of methylene blue G1 ≤ 60 μm, 60 ≤ G2 ≤ 80 μm, 80 ≤ G3 ≤ 100 μm. The initial concentration used of BM is: C0=10 mg/l, the mass of the material is: m=0.3 g and the stirring speed is: V=800 rpm. The Figure 2 shows the results obtained [8].
Figure 2: The effect of the particle size of the sawdust on the elimination of the BM Condition: C0=10 mg/l, T=(22 ± 2°C), m=0.3 g, V=800 rpm.
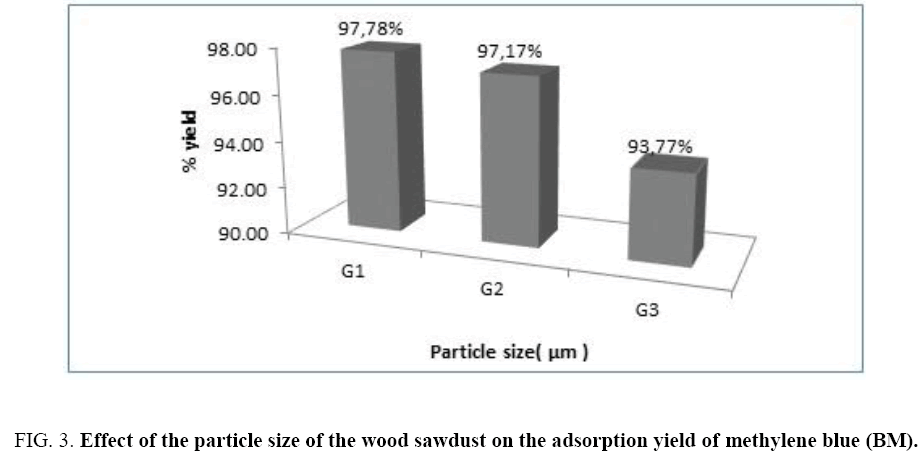
This figure shows that the balance of adsorption of the methylene blue is quickly reached for the finest particle size; G1=60 μm. The histogram represented on the Figure 3 gives the evolution of the adsorption yield according to the particle size of the wood sawdust. We notice that the maximal yield is 97.78% for the finest particle size, then this yield decreases when the particle size increases.
Figure 3: Effect of the particle size of the wood sawdust on the adsorption yield of methylene blue (BM).
We conclude that when the particle size of the beech wood sawdust is small, the adsorption is better; this fact due that the adsorption depends on the external surface of the adsorbent material which increases with the finest particle size (Figure 4).
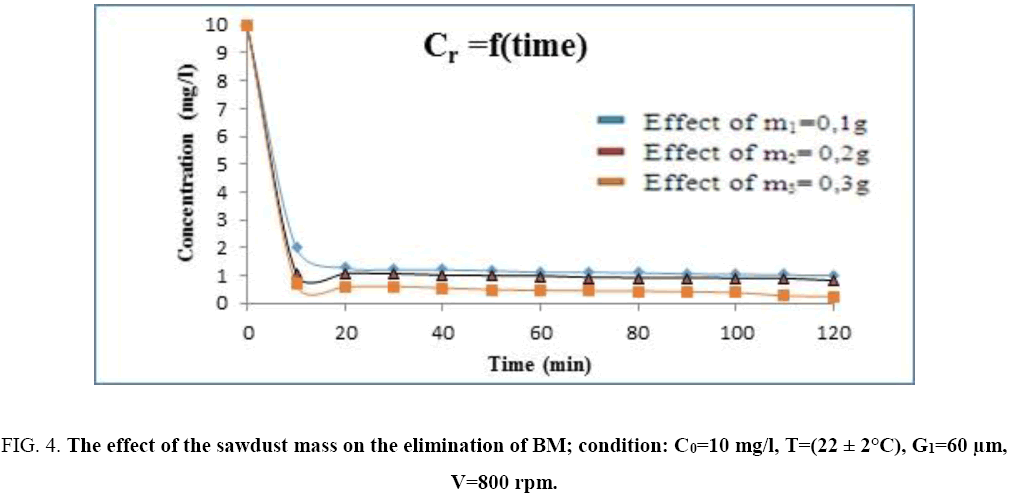
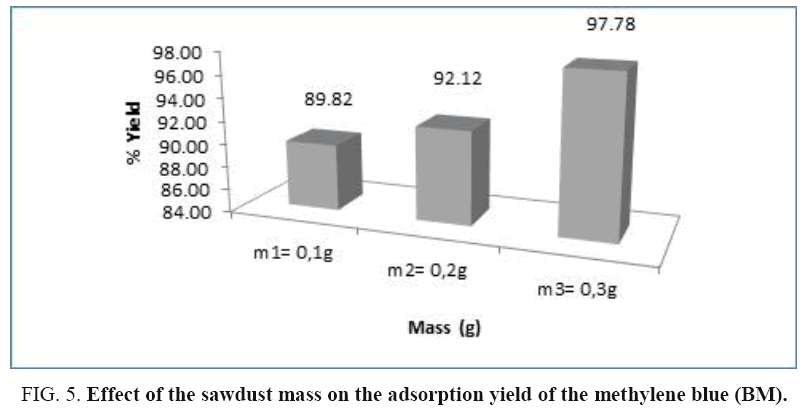
Figure 4: The effect of the sawdust mass on the elimination of BM; condition: C0=10 mg/l, T=(22 ± 2°C), G1=60 μm, V=800 rpm.
Effect of adsorbent mass: The study of the effect of the sawdust mass on the adsorption of the methylene blue had been realized by following the evolution of its residual concentration noted Cr according to time (min) in the following conditions: A particle size G=60 μm, the initial concentration of the BM is: 10 mg/l and the stirring speed is: V=800 rpm. For the adsorbent mass we have chosen three different types of mass: m1=0.1 g, m2=0.2 g and m3=0.3 g.
According to the above results, the methylene blue had been totally eliminated from the solution by a relatively important the wood sawdust mass: m=0.3 g, and during a relatively short time. The histogram below gives the evolution of the adsorption yield of (BM) according to the sawdust mass (Figure 5).
The examination of this histogram shows that the yield adsorption of the (BM) increases as the sawdust mass increases.
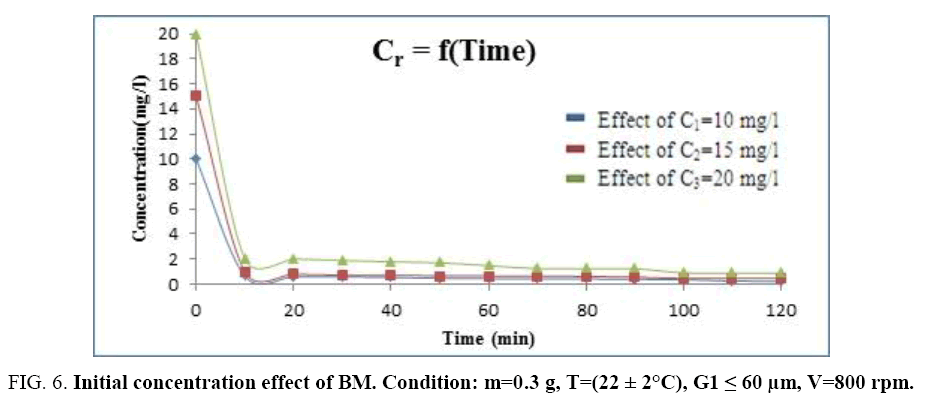
Initial concentration effect of dye and the contact time: So as to study the initial concentration effect of methylene blue on its adsorption, we should fix the adsorbent material mass in m=0.3 g, we take the finest particle size (G=60 μm) and the stirring speed is: V=800 rpm. The experiments results are shown on the Figure 6 for the various initial concentrations of the BM: C1=10 mg/l, C2=15 mg/l, C3=20 mg/l.
Figure 6: Initial concentration effect of BM. Condition: m=0.3 g, T=(22 ± 2°C), G1 ≤ 60 μm, V=800 rpm.
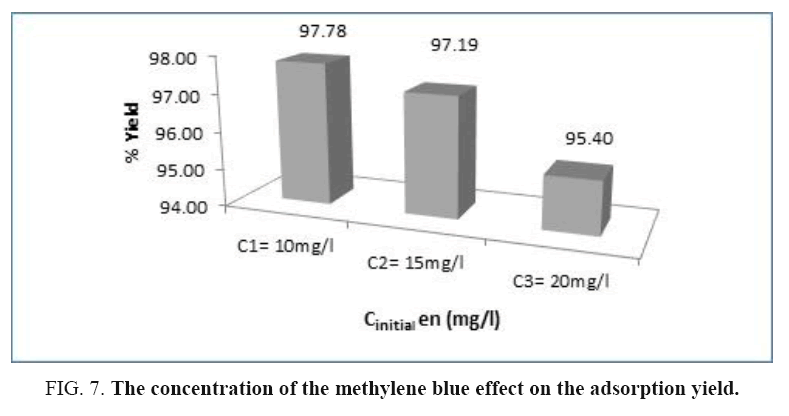
The concentrations of the BM used are chosen according of the sawdust mass so that they do not come to disrupt the phenomenon of adsorption. The Figure 6 shows a decrease of the residual concentration according to time until reach an approximately constant value at forty minutes whatever the initial concentration; this shows that this equilibrium time is independent from the initial concentration of the dye. Thus, we can conclude that the BM concentration should not exceed 20 mg/l so that the adsorption is better (Figure 7). The histogram below gives the evolution of the adsorption yield of the (BM) according to the concentration of methylene blue.
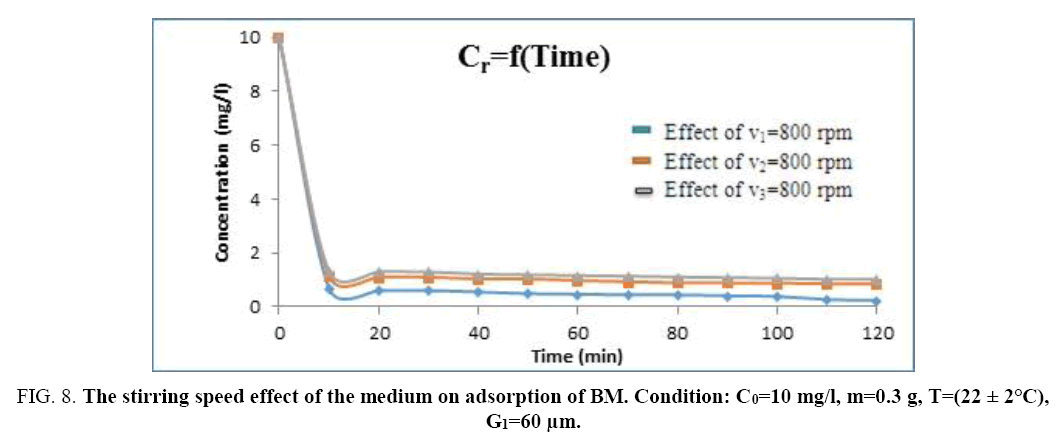
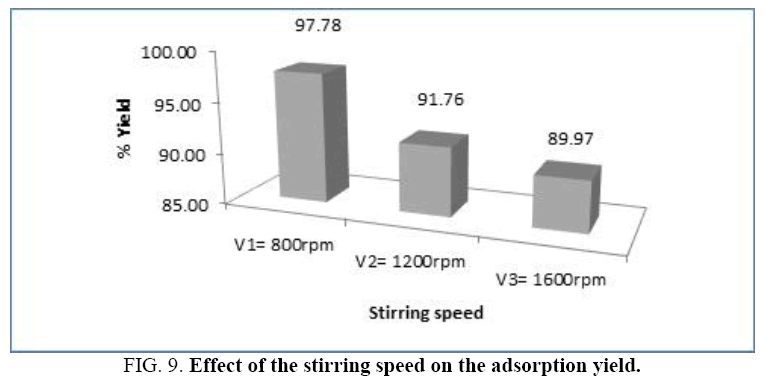
Effect of stirring speed: The phenomena of matter transfer are conditioned by the hydrodynamic conditions of the flow; this influence is more important and complex when the system is: liquid-solid type. So as to study the stirring speed effect of the medium on adsorption of BM by the sawdust, we fix the material adsorbent mass in m=0.3 g, we take the finest particle size (G=60 μm) and the initial concentration of the solution is: C=10 mg/l. The results of the experiments are shown on the Figure 8 for three levels of the stirring speed of the medium: v1=800 rpm, v2=1200 rpm, v3=1600 rpm (Figure 9).
Figure 8: The stirring speed effect of the medium on adsorption of BM. Condition: C0=10 mg/l, m=0.3 g, T=(22 ± 2°C), G1=60 μm.
The examination of this histogram shows that the adsorption yield of the (BM) decreases as the concentration of the methylene blue increases.
The treatment of results obtained shows that the quantity adsorbed by the dye is very important for a low stirring speed.
The analysis of this histogram shows that the adsorption yield of the (BM) decreases as the stirring speed of the medium increases.
Conclusion
In this study, we have processed the adsorption phenomenon of the dye-methylene blue in aqueous medium on the beech sawdust. We studied first of all experimentally the adsorption kinetic of the methylene blue (BM) on this sawdust and we envisaged the effects of: the particle size of the support, the initial concentration by dye, the adsorbent mass and the stirring speed of the medium.
The results of this study show that the adsorption yield increases in the following cases:
-The adsorbents mass is big, which suggests that the adsorption increases with the adsorbent specific surface.
-The particle size of adsorbents is fine.
-The stirring speed of medium is low.
-The initial concentration in BM is low.
The following table summarizes these optimal conditions (Table 1):
| Factors | Level | Yield (%) |
|---|---|---|
| Initial concentration of dye | C1=10 mg/l | 97.78% |
| Adsorbent mass | m2=0.3g | |
| Stirring speed of medium | v1=800 rpm | |
| Particle size | G1=60 µm |
Table 1: Optimal conditions for increasing adsorption yield.
References
- Tan IAW, HameedBH. Equilibrium and kinetic studies on basic dye adsorption by oil palm fiber activated carbon.ChemEng J. 2007;127:111-9.
- Lisheng Z,Dobias B. A method of cationic activator-aluminum salt coagulation combined with electro-floatation in treatment of waste dyeing water. Water Treatment.1992;7:221-32.
- Bagane M, Guiza S. Elimination d’un colorant des Effluents de l’industrie textile par adsorption. Ann Chim Fr. 2000;25:615-25.
- Guiza S, Bagane M, Soudani A, et al. Adsorption of basic dyes onto natural clay.AdsorpSci Tech. 2004;22(3):245-55.
- El Geundi MS. Branched-pore kinetic model for basic dyestuff adsorption onto natural clay.AdsorpSci Technol. 1992;9(3):199-211.
- El Geundi MS. Adsorbents for industrial pollution control. AdsorpSci Tech. 1977;15(10):777-87.
- Fatima O, Mohamed EK, Mohamed T, et al. Adsorption of methylene blue onto Artichoke Waste. Orient JChem. 2015;31(4):2037-41.
- Moubarak F, Atmani R, Maghri I, et al. Elimination of methylene blue dye with natural adsorbent banana peels powder. Global J Sci Front Res: B Chem.2014;14(1).