Editorial
, Volume: 15( 10) DOI: 10.37532/1753-0431.2020.15(10).1-21Evaluation of Adsorption and Absorption Factors for Radionuclides and Organic Compounds for Marine Ecosystem in Red sea , Egypt
- *Correspondence:
- Khaled. M. Zakaria Faculty of science, Al- Azhar University, Oceanography & fisheries sector, Egypt, E-mail: drkhaledzakaria@gmail.com
Received: 01-September-2020, Manuscript No. tsoc-23-97669; Editor assigned: 03- September-2020, PreQC No. tsoc-2397669 (PQ); Reviewed: 17-September-2020, QC No. tsoc-23-97669 (Q); Revised: 15-October-2020, Manuscript No. tsoc-23-97669 (R); Published: 25-October-2020, DOI: 10.37532/1753-0431.2020.15(10).1-21
Citation: Zakaria K.M, Evaluation of Adsorption and Absorption Factors for Radionuclides and Organic Compounds for Marine Ecosystem in Red sea , Egypt .Org Chem.: Indian J. 2020;15(10):1-21.
Abstract
Keywords
Radionuclides, Oil spills; Absorption; Adsorption, Organic compounds
Introduction
Naturally, radionuclides are unstable when they undergo radioactive decay become stable [1]. Recently, the number of radionuclides such as 238U, 232Th, 228Ra, and 40K in the marine biosphere has drawn much attention as a major ecological and public health concern because radium takes a long time to degrade in the human body[2]. In reality, radium-228 (228Ra) emits beta radiation with low energy and relatively poor ionization capacity, and radium226Ra (226RaRa) releases alpha particles with very high energy[3]. Several communities of the world take marine biota as a significant source of protein[4]. The three main process uptake mechanisms of radium in marine organisms are adsorption, absorption, and intake through water and feed. The fish take radionuclides through food sediment and mainly water[5]. Several researchers have demonstrated that organisms living in or on polluted sediments can bioaccumulate the pollutants [6]. An elegant experiment carried out by Farrington involving a multiphase experimental exposure design of a demersal fish (Leiostomus xanthurus) feeding on a polychaete (Nereis virens) with both fish and polychaete exposed to PCB-contaminated 77]. This factor enables the bioaccumulation abilities of two species toward a single radionuclide to be compared. In this case, the term 'bioaccumulation ability' should be understood as the relationship between the bioaccumulation rate during a given time interval and the bioaccumulative capacity. However, more than the simple measurement of radionuclide concentrations is required to distinguish which of these two components is the most influential on the final result.
In knowledge, fishes have many health benefits due to their rich source of vitamins and minerals[8]. In contrast, the intake of accumulated radionuclides fish has many serious health concerns[9]. In studies of the behavior of heavy metals and organic pollutants, radionuclides can be utilized as radiotracers[10]. “Unfortunately, in the current study, the steady state was not achieved during the experiment to calculate radionuclide concentrations in the seawater”. Therefore, it was hypothesized to measure the bioconcentration of radionuclides, total petroleum hydrocarbon, and total aliphatic hydrocarbons in marine algae with water and sediments of habitat. This could be helpful in the determination of targeted chemicals to assess the risk of a hazardous effect on health.
Materials and Methods
Study area
The study area was along the coast of Ras Ghareb, Egypt. The spatial position of station 1 is latitude 28° 21' 13ʺ and longitude 33°5' 40ʺ while station 2 is latitude 28°20' 19ʺ and longitude 33°6' 45ʺ.
Sampling
The samples were taken from superficial shoreline sediment, seawater, marine species of algae, Sargassum dentifolium (Brown algae), and Chondria seticulosa (Red algae) at Ras Ghareb, Egypt station1, and station2. The samples were properly packed in polythene bags and transported into a laboratory for chemical analysis.
Preparation of experimental samples
The seashore sediment samples were air-dried at room temperature for a week. Further, it was incubated in the oven at 80°C for 48 hours till constant dry weight was obtained while crushed and homogenized. Then the samples were milled, sieved through 0.4 mm mesh filters, and stored for further analysis. The water was collected through a water sampler of 5 liters in each separate polythene bag. Then samples were acidified with Nitric acid that attained a pH of less than 2 to avoid micro-organisms growth. The samples were stored for radioactivity and Polycyclic aromatic hydrocarbon measurements. The samples were taken out of the ice pack, thawed, and rinsed with tap water to eliminate contamination in the lab. Then it was divided into pieces and put in a sample vial after being processed with an aseptic tool and plates. After that, the samples were labeled. The samples of marine organisms Sargassum dentifolium and Chondria seticulosa were transported to the laboratory in ice boxes and stored at -10oC until further analysis, and about 20 samples of each species were collected from the study area from the same location as water samples. The marine samples were washed and cut into smaller pieces for effective grinding. The cleaned samples were dried in an oven at 70oC for five days to ensure that the sample was completely moisture free and had a constant dry weight gain. The dried samples were ground to fine grain sizes using a stainless-steel cutter blender and sieved to obtain homogeneity. All homogenized samples were divided into two parts. The first part was transferred into a 250 ml sizes Marinelli beaker that was sealed hermetically and left for about four weeks at room temperature to attain secular equilibrium among the 238U-series and 232Th-series precursors with their short-lived progenies[11]. The second part was kept in the laboratory at room temperature to be used for the analysis of the organic hydrocarbon samples.
Radioactivity measurements
The concentration of natural radioactive elements 238U, 232Th, and 40K in the samples was determined using a highresolution HPGe γ-spectrometry system with 30% counting efficiency.
It was performed using 250 cm3 counting vials filled to a height of 7 cm, corresponding to 170 cm3. The duration measurement was up to 80,000 sec and was carried out in the Egyptian Nuclear and Radiological Regulatory Authority Laboratory. The obtained spectra were analyzed. The gamma-ray transitions were used to determine the existence of radionuclides and calculate their activities. The 226RaRa or 238U activities were estimated from 234Th (92.38keV, 5.6%) for samples assumed to be in radioactive equilibrium. However, γ-energies of 214Pb (351.9 keV, 35.8%) and 214Bi (609.3,45%), (1764.5 keV, 17%), and 226RaRa (185.99 KeV, 3.5%) were used to estimate the concentration of 226RaRa. The Gamma-ray energies of 212Pb (238.6 keV, 45%), and 228Ac (338.4 keV, 12.3%), (911.07 keV, 29%), (968.90 keV, 17 %) were used to estimate the concentration of 232Th. The activity concentrations of 40K were measured directly by their gamma rays (1460.8 keV, 10.7%). An empty polystyrene container was counted the same way as the samples to assess the background distribution caused by naturally occurring radionuclides in the area around the detector. The final activity concentrations were calculated after measurement and subtraction of the background. The activities were determined by measuring their respective decay daughters [12-15].
Total Petroleum Hydrocarbons (TPH extraction )
The cute parts of algae were crushed in a mortar with a pestle. The extraction was performed using ten grams of samples from each specie, weight through analytical balance. The sample was put into a 100 mL beaker and 60mL of acetone and dichloromethane (1:1 v/v) were used as an extraction solvent. The total petroleum hydrocarbon contents of Sargassum dentifolium and Chondria seticulosa were extracted by shaking. The beaker with the content was placed on a magnetic stirrer/ heater and shaken for about 10 minutes at 70°C. The extract was poured into a clean roundbottom flask. Then 30 mL of fresh solvent was added, and the process was repeated. The extracts were combined, and 5 grams of anhydrous sodium sulfate was added to remove water. The extract was concentrated to 3 mL with a rotary evaporator maintained at 20°C. Then 1.5 mL of the concentrated extract was loaded on a silica gel column. The silica gel column was prepared by loading a 2 g glass wool, followed by 30 g silica gel, onto a chromatographic column that was 2 cm internal diameter and 10 cm long. Each bed was prepared with 40 ml HPLC-hexane to remove any organic contaminant.
Furthermore, 1.5 mL concentrated extract was loaded and eluted with 30 mL HPLC hexane into a labeled100 mL beaker to get the aliphatic hydrocarbon components in the sample. After the hexane had almost eluted through the column, but before completely letting the column dry, 30 mL of dichloromethane was added to elute the aromatic hydrocarbon contents into another labeled 100 mL beaker. Then 2 g of anhydrous sodium sulfate was added to remove any traces of water left in the extract. The fractions were concentrated using a rotary evaporator of about 2 ml. Then 1ml of the extract was transferred into a well-labeled vial ready for gas chromatographic analysis. The samples were stored at 4°C until GC analysis.
Gas chromatographic analysis
Each extract was transferred to a 1.5 mL vial and was loaded into a gas chromatography system Agilent 6890 series model G1530 A, with Flame Ionization Detector (FID) and cold on-column injection. Then 1μL sample was injected and analyzed for TPH (C9–C36). An HP-5 (crosslinked PH ME siloxane) column with dimensions 30 m x 0.25 mm with a stationary phase thickness of 0.25 μm was used for analytical separation. The carrier gas was purified nitrogen held at a flow rate of 5 mL per minute. The operating temperature program was started at 60°C for 2 mins and then increased at a rate of 10°C per minute to 300°C for 10 minutes. The injector and detector temperature were maintained at 250°C(FIG. 1).
Results and Discussion
Radionuclides in seawater and sediments
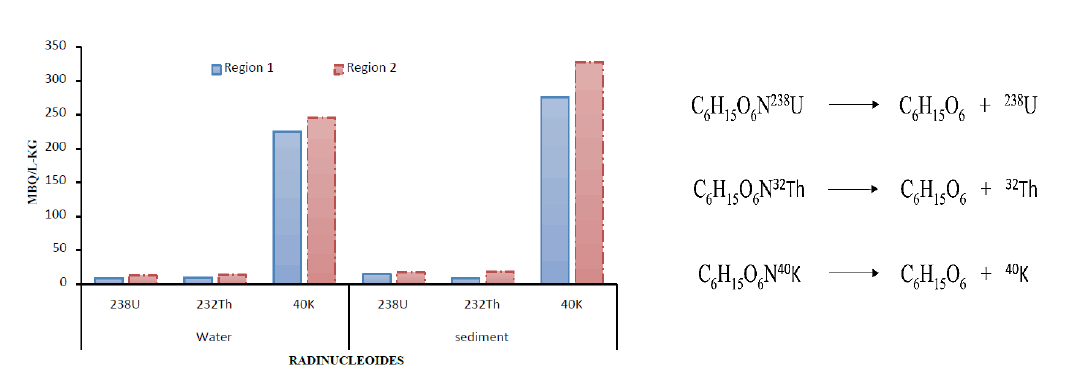
The absorption of 238U, 232Th, and 40K in seawater and coastal sediments of two regions in Ras Ghareb, Egypt. nucleoid concentration was found higher in sediments than in seawater of both regions (FIG. 2). Thorium was found with low concentration in sediments than water only in the region1. While the amount of 40K in both water and sediments varies significantly (TABLE 1).
TABLE 1. Concentration of 238U, 232Th, and 40K in seawater and coastal sediments collected from different locations along the Ras Ghareb region coastline.
| Radionuclide's | Sites | |||
|---|---|---|---|---|
| Ras Ghareb Station 1 | Ras Ghareb Station 2 | |||
| Latitude 28° 21´ 13ʺ |
Longitude 33° 5´ 40ʺ |
Latitude 28° 20´ 19ʺ |
Longitude 33° 6´ 45ʺ |
|
| Water (mBq/L) |
Sediment (Bq/kg-1) |
Water (mBq/L) |
Sediment (Bq/kg-1) |
|
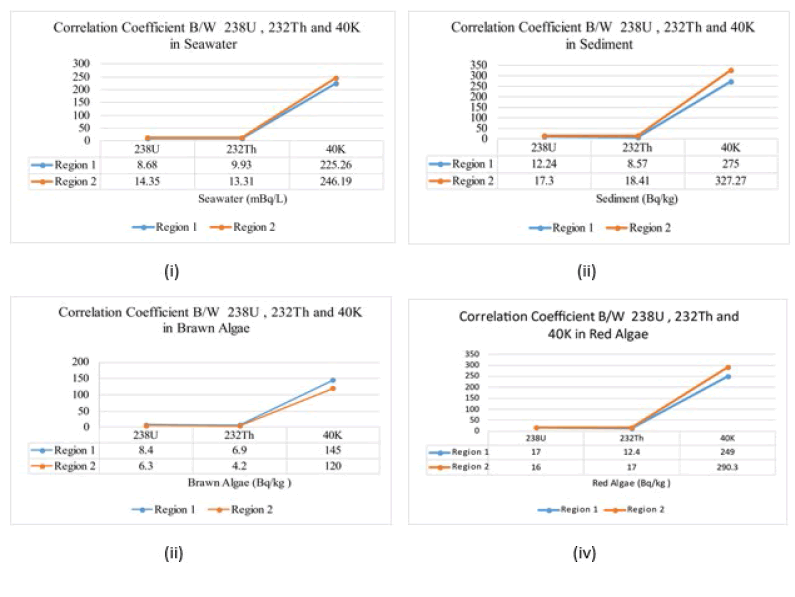
| 238U | 8.68±0.12 | 12.24±0.13 | 14.35±0.14 | 17.3±0.15 |
| 232Th | 9.93 ±0.11 | 8.57±0.35 | 13.31±0.35 | 18.41±0.38 |
| 40K | 225.26±2.7 | 275.26±2.4 | 246.19±1.9 | 327.27±3.1 |
A study was done along the coast of the Oman Sea on the concentration of 238U, 232Th, and 40K, showing that sediments have agreater variety of radionuclides than water samples. Though, it was acceptable in terms of environmental andradioisotope risks when compared to reference values fromIran and other regions of the world. Regarding the turkey, the radio nucleotide value was found higher than permitted by global guidelines[16].
Another study in the Gulf of Thailand of these three natural radionuclides found high concentrations in marine sediment. In Jeddah, Saudi Arabia, a similar study found the normal limit of these radionuclides in the sediment.
Radionuclides in marine biota
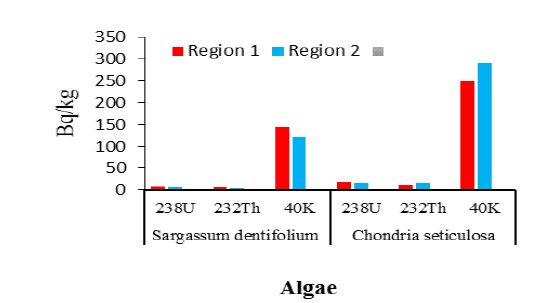
The comparative analysis of radioactive material in both species revealed varying levels in their respective locations (FIG. 3). The concentration of selected radionuclides was found high in Chondria seticulosa in both regions. While the concentration of these radionuclides was high in region 1 than in region 2 in both algae species (TABLE 2).
Figure 3: Bioconcentration of Radionuclides in algae species of region 2.
TABLE 2. Concentration of 238U, 232Th, and 40K in marine organisms collected from different locations along the Ras Ghareb region coastline.
| Radionuclide's | Sites | |||
|---|---|---|---|---|
| Ras Ghareb region 1 | Ras Ghareb region 2 | |||
| Sargassum dentifolium Brawn algae (Bq/kg ) |
Chondria seticulosa Red algae (Bq/kg ) |
Sargassum dentifolium Brawn algae (Bq/kg ) |
Chondria seticulosa Red algae (Bq/kg ) |
|
| 238U | 8 . 4 ±0.1 4 | 1 7 . 5 ±0. 16 | 6 . 3 ±0. 13 | 16. 1 ±0.2 4 |
| 232Th | 6 .9 ±0.11 | 12. 4 ±0. 26 | 4 .2±0. 1 5 | 17 . 4 ±0. 21 |
| 40K | 1 45.18±1.7 | 249 .7± 2 . 8 | 12 0. 8± 1 . 6 | 290 . 31 ± 2 . 5 |
| 238U: Isotope of uranium, 232Th: Naturally occurring isotope of thorium, 40K: Radioactive isotope of potassium, Bq/kg-1: Becquerel per kilogram | ||||
A research study was conducted in Nigeria to find out the concentration of radionuclides in fish and fish feed, due to their high consumption rate in the area. The results found that both adults and children received annual committed effective doses that were all below the suggested 1.0 mSv y1 limit for members of the public. This demonstrates that, from a radiological standpoint, the radiation dose derived from ingesting fresh fish samples does not represent any substantial health hazards to the public [17, 18]. An investigation was made into the radioactive activity of wild fungi in Iraq, particularly black desert truffles. The measured values were identified as lower than the estimated value for the international mean [19].
The bioconcentration factor algae
The bioconcentration of radionuclides was found with a high concentration in Chondria seticulosa in both regions of the samples. The high concretion of 238U 2.016, was found in Chondria seticulosa in Region1. While the BCF of 40K was 1.178 in Chondria seticulosa The use of concentration factors as a practical technique to represent the accumulation of radionuclides in biota relative to radionuclide concentrations in seawater was motivated by the necessity to report radionuclide accumulation in biota in various conditions and geographical areas. Later, concentration factors were used to forecast radioactivity in organisms and model radioactive distribution and transfer in aquatic settings [20]. In China, it was found that humans ingested the aquatic animals at a determined committed effective dose of 0.06 mSv-2.99 mSv. The artificial nuclides 90Sr and 137Cs had minimal dose contributions, but 210Po was the main source of radiation damage in both marine creatures and humans [21].
Petroleum hydrocarbons in seawater and sediments
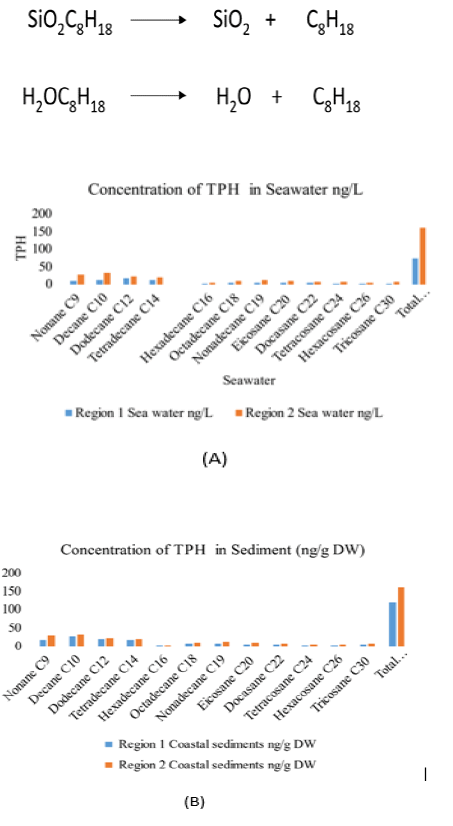
The findings of TPH show varying results in seawater and sediments of both sites. The highest value of Decane C10 was 31.6 ng/g DW followed by N (TABLE 3).
TABLE 3. Bioconcentration Factor (BCF) for radionuclides in marine organisms along the Ras Ghareb region coastline.
| Radionuclide's | Sites | |||
|---|---|---|---|---|
| Ras Ghareb region 1 | Ras Ghareb region 2 | |||
| Sargassum dentifolium Brawn algae |
Chondria seticulosa Red algae |
Sargassum dentifolium Brawn algae |
Chondria seticulosa Red algae |
|
| BCF 238U | 0.967 | 2.016 | 0.439 | 1.121 |
| BCF 232Th | 0.694 | 1.248 | 0.315 | 1.307 |
| BCF 40K | 0.644 | 1.108 | 0.490 | 1.178 |
| 238U: Isotope of uranium, 232Th: Naturally occurring isotope of thorium, 40K: Radioactive isotope of potassium, BCF: Bio-Concentration Factor | ||||
Nonane C9 at 28.3 ng/g DW was found in Region 2 of Ras Ghareb among the TPH. The overall values of TPH were observed high in region 2 as compared to Region 1 (FIG. 4). The TPH 160.2 ng/g DW was found in the Region 2 coastal sediments (TABLE 4).
TABLE 4. Concentration levels of total aliphatic hydrocarbons TAH found in marine organisms collected from different locations along the Ras Ghareb region coastline.
| TPH | Sites | |||
|---|---|---|---|---|
| Ras Ghareb region 1 | Ras Ghareb region 2 | |||
| Sea water ng/L | Coastal sediments ng/g DW | Sea water ng/L | Coastal sediments ng/g DW | |
| Nonane C9 | 9 . 4 | 18.3 | 21.4 | 28.3 |
| Decane C10 | 12 . 6 | 26.1 | 26.9 | 31.6 |
| Dodecane C12 | 17 . 5 | 19.3 | 18.2 | 22.4 |
| Tetradecane C14 | 11 . 9 | 17.9 | 14.6 | 19.3 |
| Hexadecane C16 | 0.9 | 2.7 | 1.9 | 3.6 |
| Octadecane C18 | 4.7 | 6.8 | 8.6 | 9.4 |
| Nonadecane C19 | 3.2 | 7.4 | 9.4 | 11.6 |
| Eicosane C20 | 2.8 | 4.6 | 7.2 | 9.3 |
| Docasane C22 | 4.2 | 6.2 | 6.5 | 7.2 |
| Tetracosane C24 | 1.3 | 3.1 | 4.4 | 6.1 |
| Hexacosane C26 | 1.1 | 2.9 | 2.7 | 4.6 |
| Tricosane C30 | 2.4 | 4.6 | 5.8 | 6.8 |
| Total Petroleum hydrocarbon | 72 | 119.9 | 127.6 | 160.2 |
| ng/L: Nanogram per litre, ng/g DW: Nanogram per gram of dry weight | ||||
According to a study in the Southern China sea on TPHs and n-alkanes. Here it was found that TPHs were higher than those on the far shore in the central and northern waters along the shoreline. The concentration of n-alkanes in the water samples ranged from C10 to C38, and they were primarily produced by higher terrestrial plants [22]. The TPH levels found in Pulicat Lake in India throughout the study period present less of an ecological threat to the environment and biota [23].
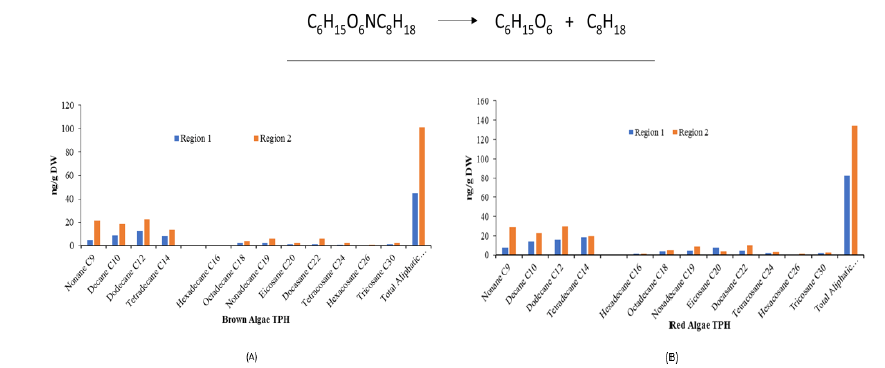
Total aliphatic hydrocarbons in brown and red algae
The results of TAH demonstrate that both sites of water and sediments have different outcomes. In Region 2 of Ras Ghareb, among the TAH, the highest concentration of Dodecane C12 was found at 29.7 ng/g DW, followed by Nonane C9 at 28.2 ng/g DW of Chondria seticulosa. When comparing the TAH of area 1 which was 45.5 and 82.7 with region 2 which was 101 and 134, it was found that region 2 has a high concentration of aliphatic hydrocarbons (FIG. 5). Correspondingly, it was also observed that Chondria seticulosa has a high absorption capacity of TAH than Sargassum dentifolium (TABLE 5). The F3 and F4 fractions of Fucus viroids were found to have good radical scavenging properties in vitro, and zebrafish embryos showed a protective effect against oxidative stress brought on by hydrogen peroxide (FIG. 6) [24]. The two species of algae Taonia atomaria and Padina pavonica of the central Adriatic Sea were significantly found to be primary oil composition constituents[25] (TABLE 6) .
TABLE 5. Concentration levels of total aliphatic hydrocarbons TAH found in marine organisms collected from different locations along the Ras Ghareb region coastline.
| TAH | Sites | |||
|---|---|---|---|---|
| Ras Gharebregion 1 | Ras Gharebregion 2 | |||
| Sargassum dentifolium Brawn algae ng/g DW |
Chondria seticulosa Red algae ng/g DW |
Sargassum dentifolium Brown algae ng/g DW |
Chondria seticulosa Red algae ng/g DW |
|
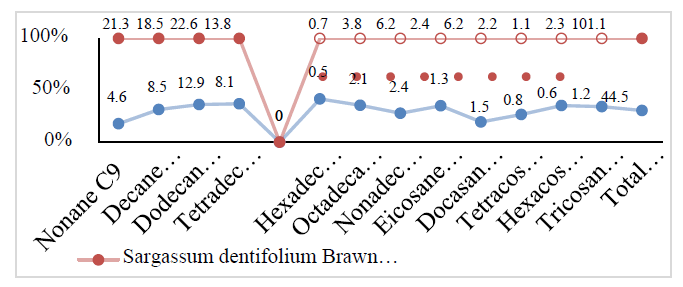
| Nonane C9 | 4.6 | 7.3 | 21.3 | 28.4 |
| Decane C10 | 8.5 | 14.2 | 18.5 | 22.3 |
| Dodecane C12 | 12.9 | 15.8 | 22.6 | 29.7 |
| Tetradecane C14 | 8.1 | 18.3 | 13.8 | 19.4 |
| Hexadecane C16 | 0.5 | 1.1 | 0.7 | 1.2 |
| Octadecane C18 | 2.1 | 3.8 | 3.8 | 4.9 |
| Nonadecane C19 | 2.4 | 4.1 | 6.2 | 8.2 |
| Eicosane C20 | 1.3 | 7.3 | 2.4 | 3.8 |
| Docasane C22 | 1.5 | 4.2 | 6.2 | 9.6 |
| Tetracosane C24 | 0.8 | 1.7 | 2.2 | 3.4 |
| Hexacosane C26 | 0.6 | 0.9 | 1.1 | 1.3 |
| Tricosane C30 | 1.2 | 1.9 | 2.3 | 2.6 |
| Total Aliphatic hydrocarbon | 44.5 | 82.7 | 101.1 | 134.8 |
| ng/L: nanogram per litre, ng/g DW: nanogram per gram of dry weight. | ||||
Figure 6: The correlation of absorption and adsorption of 238U, 232Th, 40 K in i) Seawater, ii) Sediments iii) Brawn algae iv) Red algae.
TABLE 6. Bio-Concentration Factor (BCF) for the investigated TAH in the investigated marine organisms in the water.
| BCF TAG |
Sites |
|||
|---|---|---|---|---|
| Ras Ghareb region 1 | Ras Ghareb region 2 | |||
| Sargassum dentifolium Brawn algae |
Chondria seticulosa Red algae |
Sargassum dentifolium Brawn algae |
Chondria seticulosa Red algae |
|
| Nonane C9 | 0.489 | 0.776 | 0.995 | 1.327 |
| Decane C10 | 0.674 | 1.12 | 0.687 | 0.828 |
| Dodecane C12 | 0.737 | 0.902 | 1.241 | 1.631 |
| Tetradecane C14 | 0.680 | 1.537 | 0.945 | 1.328 |
| Hexadecane C16 | 0.555 | 1.222 | 0.368 | 0.631 |
| Octadecane C18 | 0.446 | 0.808 | 0.441 | 0.569 |
| Nonadecane C19 | 0.75 | 1.281 | 0.659 | 0.872 |
| Eicosane C20 | 0.464 | 2.607 | 0.333 | 0.521 |
| Docasane C22 | 0.357 | 1 | 0.953 | 1.476 |
| Tetracosane C24 | 0.615 | 1.307 | 0.5 | 0.772 |
| Hexacosane C26 | 0.545 | 0.818 | 0.407 | 0.481 |
| Tricosane C30 | 0.5 | 0.791 | 0.396 | 0.448 |
| BCF Total Aliphatic hydrocarbon | 6.812 | 14.169 | 7.29 | 10.884 |
| BCF: Bio-Concentration Factor, TAH: Total Aliphatic Hydrocarbons | ||||
Bioconcentration factor for total aliphatic hydrocarbons in brown and red algae
The BCF of Region 1 6.8, 14.7 has greater than region 2, while Chondria seticulosa was shown a higher value of TAH in both sites. Eicosane C20 was the highest BCF in Chondria seticulosa of Region 1. The high bioconcentration of aliphatic compounds in Chondria seticulosa indicates its absorption capacity(TABLE 7).
TABLE 7. Comparison of activity concentration of 226Ra, 232Th, and 40K (Bq/kg) in algae in other studies.
| Location | 238U Series Bq/kg | 232Th Series Bq/ kg | 40K Bq/ kg | Reference |
|---|---|---|---|---|
| Nigeria | 33 | 45 | 420 | |
| Egypt | 8 . 4 | 6 .9 | 1 45.18 | Present study |
The ability of mangroves to absorb and store heavy metals in their tissue lower heavy metals in the aquatic environment because of their capacity to do so (FIG. 7). According to the review, the mangrove species have a promising potential to be employed for biomonitoring in the aquatic environment[26-32] (TABLE 8). Perennial herbs species were identified as sensitive that can be produced to lessen soil contamination in Pazanan, based on their frequency and resistance to adverse conditions is a suitable option for the phytoremediation of soil contaminated with nickel and TPHs [33-44] (TABLE 9) .
TABLE 8. Comparison of activity concentration of 226Ra, 232Th, and 40K (Bq/L) in Seawater those in other studies.
| Location | 238U Series Bq/l | 232Th Series Bq/l | 40K Bq/l |
|---|---|---|---|
| Egypt | 0.971– 1.6 | 0.21– 1.1 | 0.97–23 |
| Iran | 0. 53 | 2.08 | 7. 17 |
| Jordon | 3. 7 | 2.41 | 24. 20 |
| Pakistan | 0.00175 | 0.00235 | 0.04708 |
| Yemen | 3. 47 | 2.02 | 15. 05 |
| Turkey | 0. 72 | 0. 53 | 2. 40 |
| Iraq (Nineveh province) | 0.842 | 0.93 | 25.92 |
| Present work | 8.68 ± 0.12 | 9.93 ± 0.11 | 225.26 ± 2.7 |
TABLE 9. Comparison of activity concentration of 226Ra, 232Th, and 40K (Bq/ Kg) in Sediments those in other studies.
| Location | 238U Bq/Kg | 232Th Bq/ Kg | 40K Bq/ Kg |
|---|---|---|---|
| World average | 32 | 45 | 412 |
| Egypt (Gulf of Suez) | 13.79 | 14.55 | 128.67 |
| Egypt (Red Sea) | 23.80* | 19.60 | 374.90 |
| Egypt (Mediterranean Sea) | 8.80 * | 30.80 | 106.9 |
| Oman | 20.49 | 2.26 | 44.83 |
| Iran (Caspian Sea) | 34.40* | 11.40 | 310.00 |
| Serbia (Boka Kotorska Bay) | 37.00 | 35.00 | 580.00 |
| Cyprus (East coast region) | 23.00* | 19.00 | 628.10 |
| China (Beibu Gulf) | 25.90 | 37.6 | 263 |
| India (Tamilnadu) | 47.04 | 26.63 | 372.49 |
| Bangladesh (Bay of Bengal) | 31.20 | 51.90 | 686.40 |
| Turkey (Kocaeli- black sea) | 8.85 | 8.93 | 219.41 |
| Ghana (Tema Harbour) | 34.00 | 30.00 | 320.00 |
| Nigeria (Akwa Ibom) | 23.00 | 36.00 | 145.00 |
| Turkey | 25.50 ± 21.50 | 27.90 ± 2.40 | 590.30 ± 28.60 |
| Bangladesh | 28.67 ± 3.09 | 49.46 ± 3.58 | 560.87 ± 81.40 |
| Malaysia | 41.00 ± 2.00 | 45.00 ± 4.00 | 680.00 ± 59.00 |
| Saudi Arabia | 26.40 ± 2.80 | 16.30 ± 2.20 | 451.00 ± 15.00 |
| Oman | 22.68 ± 0.32 | 21.38 ± 0.37 | 222.89 ± 3.52 |
| Indonesia | 47.29 ± 4.14 | 52.73 ± 5.28 | 744.00 ± 29.45 |
| Saudi Arabia | 3.50* | 5.90** | 113.50 |
| Present study | 12.24 ± 0.13 | 8.57 ± 0.35 | 275.26 ± 2.4 |
Investigated marine organisms 238U: Isotope of uranium, 232Th: naturally occurring isotope of thorium, 40K: radioactive isotope of potassium, BCF: Bio-concentration factor[45-57] .
Conclusion
The chemical analysis of seawater, sediments and marine algae was performed to investigate their adsorption and absorption capacity of radionuclides, total petroleum hydrocarbons, and total aliphatic hydrocarbons. the concentration and bioaccumulation of radionuclides, TPH, and TAH were observed with high levels in the coastal sediments and red algae of Ras Gareb as compared to water and brown algae. The value of radionuclides was found within the acceptable range of international limits. The Chondria seticulosa were found with significant absorption of TPH, TAH, and radionuclides. So, it could be used to lower the concentration of these chemicals in seawater.
References
- Coura-Filho GB, Torres Silva de Oliveira M, Morais de Campos AL. Principles of Radionuclide Treatments. Cham: Springer Int. Publ. 2022:21-31
- Adeola AO, Iwuozor KO, Akpomie KG, et al. Advances in the management of radioactive wastes and radionuclide contamination in environmental compartments: a review. Environ. Geochem. Health. 2022. 13:1-27.
- Coupannec M. Optimization and Comparison of Radioanalytical Methods for the Determination of Radium and Other Alpha-Emitting Radionuclides in Process Water Samples from the Oil & Gas Industry (Doctoral dissertation, Colorado State University).
- Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022;20(1):1-38.
- Bezhenar R, Takata H, de With G, et al. Planned release of contaminated water from the Fukushima storage tanks into the ocean: Simulation scenarios of radiological impact for aquatic biota and human from seafood consumption. Mar. Pollut. Bull. 2021;173:112969.
- Rakib MR, Rahman MA, Onyena AP, et al. A comprehensive review of heavy metal pollution in the coastal areas of Bangladesh: abundance, bioaccumulation, health implications, and challenges. Environ. Sci. Pollut. Res. 2022;29(45):67532-58.
- Farrington JW. Bioaccumulation of hydrophobic organic pollutant compounds. Ecotoxicol.: probl. approaches. 1989:279-313.
- Chen J, Jayachandran M, Bai W, et al. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022;369:130874.
- Joseph A, Edet U, Etinosa-Okankan O, et al. Health risk assessment of heavy metals and radionuclides in Cynoglossus senegalensis (Sole fish) from Qua Iboe River, South-South Nigeria. J. Food Compos. Anal. 2022;114:104854.
- Hansen, S. B., & Bender, D. (2020, November). Advancement in production of radiotracers..
- Harley NH. Radon and lung cancer. Environ. Toxic.: Hum. Expo. Their Health Eff. 2020:877-909.
- Azli T, Zidouni F, Messaoudi M, et al. Gamma spectrometry for natural radioactive nuclides in Spa waters in some areas in north Algeria. Alger. J. Eng. Technol. 2021;5.
- Roviello V, De Cesare M, D'Onofrio A et al. New analytical methods for the assessment of natural (238U, 232Th, 226Ra, 40K) and anthropogenic (137Cs) radionuclides as actinides (239Pu, 240Pu): The case study of the Garigliano NPP releases along the Domitia sandy beaches (Southern Italy). Catena. 2020;193:104612.
- Kuppusamy S, Maddela NR, Megharaj M, et al. Methodologies for analysis and identification of total petroleum hydrocarbons. Total pet. hydrocarb.: environ. fate toxic. remediat. 2020:29-55.
- Lin W, Feng Y, Yu K, et al. Long-lived radionuclides in marine sediments from the Beibu Gulf, South China Sea: Spatial distribution, controlling factors, and proxy for transport pathway. Mar. Geol.. 2020 ;424:106157.
- Yorgun NY, Oto B, Gür F, et al. Measurement of the Concentration of 226Ra, 232Th, 40K in The Soil In Settlements on the Coastline of Lake Van, Turkey. Radiat. Prot. Dosim. 2022;198(20):1575-84.
- Lin W, Feng Y, Yu K, et al. Long-lived radionuclides in marine sediments from the Beibu Gulf, South China Sea: Spatial distribution, controlling factors, and proxy for transport pathway. Mar. Geol. 2020;424:106157.
- Al-Mur BA, Gad A. Radiation hazard from natural radioactivity in the marine sediment of Jeddah Coast, Red Sea, Saudi Arabia. J. Mar. Sci. Eng. 2022;10(8):1145.
- Mohammed RS, Ahmed RS. Yearly effective dose due to consumption of wild black fungus grow in southern Iraq assessed by measuring of radionuclide concentrations. InJournal Phys.: Conf. 2020;1660: 012096.
- Haghshenas V, Kafaei R, Tahmasebi R, et al. Potential of green/brown algae for monitoring of metal (loid) s pollution in the coastal seawater and sediments of the Persian Gulf: ecological and health risk assessment. Environ. Sci. Pollut. Res. 2020;27:7463-75.
- Sun J, Men W, Wang F, et al. Activity Levels of 210Po, 210Pb and Other Radionuclides (134Cs, 137Cs, 90Sr, 110mAg, 238U, 226Ra and 40K) in Marine Organisms From Coastal Waters Adjacent to Fuqing and Ningde Nuclear Power Plants (China) and Radiation Dose Assessment. Front. Mar. Sci. 2021;8:702124.
- Gong S, Liu W, Li Y, et al. Distribution characteristics and source tracing of petroleum hydrocarbons in the northeastern South China Sea. Chin. Chem. Lett. 2020;31(10):2854-8.
- Hemalatha D, Sanil S, Kumar BC, et al. Spatial distribution of total petroleum hydrocarbons in sediments of Pulicat Lake, Southeast coast of India. Environ. Chem. Ecotoxicol. 2020;2:175-81.
[Google Scholar] [Cross Ref]
- Jerković I, Cikoš AM, Babić S, et al. Bioprospecting of less-polar constituents from endemic brown macroalga Fucus virsoides J. Agardh from the Adriatic Sea and targeted antioxidant effects in vitro and in vivo (zebrafish model). Mar. drugs. 2021;19(5):235.
- Carriot N, Paix B, Greff S, et al. Integration of LC/MS-based molecular networking and classical phytochemical approach allows in-depth annotation of the metabolome of non-model organisms-The case study of the brown seaweed Taonia atomaria. Talanta. 2021;225:121925.
- Wilda, R., Hamdan, A. M., & Rahmi, R. A review: The use of mangroves for biomonitoring on the aquatic environment. In IOP Conference Series: Materials Science and Engineering 2020;980: 012083)..
- Bahrami M, Jahantab E, Mahmoudi MR. Clustering the organic soil amendments in combination with phytoremediation of heavy metals contaminated soil. Int. J. Environ. Anal. Chem. 2021:1-5.
- Kuppusamy S, Maddela NR, Megharaj M, et al. Methodologies for analysis and identification of total petroleum hydrocarbons. Total pet. hydrocarb.: environ. fate toxic. remediat. 2020:29-55.
- Tyovenda AA, Ikpughul SI, Sombo T. Assessment of radionuclides in water, sediment and algae in river Benue at Jimeta-Yola, Adamawa State, Nigeria. Annu. Natl. Conf. Niger. Inst. Phys. 2018;50:29
- Alsofy D, Al-jomaily FM. Estimation of Radionuclides Elements for Natural Water Resources in Nineveh Province, Iraq Using Gamma Ray Spectroscopy. Arab J. Nucl. Sci. Appl. 2023;56(1):99-111.
- Tawfiq NF, Mansour HL, Karim MS. Natural radioactivity in soil samples for selected regions in Baghdad governorate. Int. J. Recent Res. Rev. 2015;8(1):1-7.
- Arabi AE, Ahmed NK, Salahel Din K. Natural radionuclides and dose estimation in natural water resources from Elba protective area, Egypt. Radiat. prot. dosim.. 2006;121(3):284-92.
- Parhoudeh M, Khoshgard K, Zare MR, et al. Natural radioactivity level of 226Ra, 232Th, and 40K radionuclides in drinking water of residential areas in Kermanshah province, Iran using gamma spectroscopy. Iran. J. Med. Phys.. 2019; 16(1):98-102.
- Kashparov VA, Lundin SM, Khomutinin YV, et al. Soil contamination with 90Sr in the near zone of the Chernobyl accident. J. environ. radioact. 2001;56(3):285-98.
- Ahmad N, Khan A, Ahmad I, et al. Health implications of natural radioactivity in spring water used for drinking in Harnai, BalochistanInt. J. Environ. Anal. Chem. 2021;101(9):1302-9.
- Abd El-Mageed AI, El-Kamel AE, Abbady AE, et al. Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination. 2013;321:28-31.
- Al-Mur BA, Gad A. Radiation hazard from natural radioactivity in the marine sediment of Jeddah Coast, Red Sea, Saudi Arabia. J. Mar. Sci. Eng. 2022;10(8):1145.
- Akpan AE, Ebong ED, Ekwok SE et al. Assessment of radionuclide distribution and associated radiological hazards for soils and beach sediments of Akwa Ibom Coastline, southern Nigeria. Arab. J. Geosci.,2020:1-2.
- Botwe BO, Schirone A, Delbono I et al. Radioactivity concentrations and their radiological significance in sediments of the Tema Harbour (Greater Accra, Ghana). J. Radiat. Res. Appl. Sci.. 2017;10(1):63-71.
- Korkulu Z, Özkan N. Determination of natural radioactivity levels of beach sand samples in the black sea coast of Kocaeli (Turkey). Radiat. Phys. Chem.. 2013;88:27-31.
- Bhuiyan MK, Siddique MA, Zafar M, et al. Spatial distribution of radioisotope concentrations in the offshore water and sediment of the Bay of Bengal (Indian Ocean), BangladeshIsot. Environ. Health Stud. 2014 ;50(1):134-41.
- Thangam V, Rajalakshmi A, Chandrasekaran A et al. Determination of natural radioactivity in beach sands collected along the coastal area of Tamilnadu, India using gamma ray spectrometry. J. Radioanal. Nucl. Chem.2022 ;331(3):1207-23.
- Abbasi A, Zakaly HM, Mirekhtiary F. Baseline levels of natural radionuclides concentration in sediments East coastline of North Cyprus. Mar. Pollut. Bull.2020;161:111793.
- Radomirović M, Stanković S, Mandić M, et al. Spatial distribution, radiological risk assessment and positive matrix factorization of gamma-emitting radionuclides in the sediment of the Boka Kotorska Bay. Mar. Pollut. Bull. 2021;169:112491.
- Abbasi A, Algethami M, Bawazeer O, et al. Distribution of natural and anthropogenic radionuclides and associated radiation indices in the Southwestern coastline of Caspian Sea. Mar. Pollut. Bull.. 2022;178:113593.
- Al Shaaibi M, Ali J, Duraman N, et al. Assessment of radioactivity concentration in intertidal sediments from coastal provinces in Oman and estimation of hazard and radiation indices. Mar. Pollut. Bull. 2021;168:112442.
- Awad M, El Mezayen AM, El Azab A, et al. Radioactive risk assessment of beach sand along the coastline of Mediterranean Sea at El-Arish area, North Sinai, Egypt. Mar. Pollut. Bull. 2022 Apr 1;177:113494.
- Zakaly HM, Uosif MA, Issa SA, . An extended assessment of natural radioactivity in the sediments of the mid-region of the Egyptian Red Sea coast. Mar. Pollut. Bull.. 2021;171:112658.
- Diab HM, Ramadan A, Monged MH, et al. Environmental assessment of radionuclides levels and some heavy metals pollution along Gulf of Suez, Egypt. Environ. Sci. Pollut. Res. 2019 ;26(12):12346-58.
- United Nations Scientific Committee. UNSCEAR, sources and effects of ionization radiation, on the Effects of Atomic Radiation. Report to the General Assembly with scientific annexes. 2000;1. [Google Scholar] [Cross Ref]
- Alzahrani JS, Almuqrin A, Alghamdi H et al. Radiological monitoring in some coastal regions of the Saudi Arabian Gulf close to the Iranian Bushehr nuclear plant. Mar. Pollut. Bull.. 2022 ;175:113146.
- Mukanthi D, Jayuska A, Makmur M, Idiawati N. Kajian Kualitas Air Laut dan Dosis Cesium 137 Pada Biota di Pantai Gosong, Kalimantan Barat Sebagai Calon Tapak PLTN. Jurnal Pengembangan Energi Nuklir. 2021 Dec 31;23(2):109-17.
[Google Scholar] [Cross Ref]
- Özmen SF, Cesur A, Boztosun I, et al. Distribution of natural and anthropogenic radionuclides in beach sand samples from Mediterranean Coast of Turkey. Radiat. phys. chem.. 2014;103:37-44.
- Ahmed MM, Das SK, Haydar MA, et al. Study of natural radioactivity and radiological hazard of sand, sediment, and soil samples from Inani Beach, Cox’s Bazar, Bangladesh. J. Nucl. Part. Phys. 2014;4(2):69-78.
- Yii MW, Zaharudin A, Abdul-Kadir I. Distribution of naturally occurring radionuclides activity concentration in East Malaysian marine sediment. Appl. Radiat. Isot.. 2009 ;67(4):630-5.
- Alshahri F. Radioactivity of 226Ra, 232Th, 40K and 137Cs in beach sand and sediment near to desalination plant in eastern Saudi Arabia: assessment of radiological impacts. J. King Saud Univ.-Sci. 2017;29(2):174-81.
- Darabi-Golestan F, Hezarkhani A, Zare MR. Assessment of 226Ra, 238U, 232Th, 137Cs and 40K activities from the northern coastline of Oman Sea (water and sediments). Mar. Pollut. Bull.. 2017;118(1-2):197-205..