Original Article
, Volume: 16( 14)Estimation of Mineral Oil Contamination in Synthetic Lubricants by 1H NMR Spectroscopy
- *Correspondence:
- Sujit M, R&D Center, Indian Oil Corporation Limited, Sector-13, Faridabad-121007, India, E-mail: mondals3@indianoil.in
Received: June 17, 2016; Accepted: June 28, 2016; Published: July 06, 2016
Citation: Sujit M, Dileep K, Ravindra K, Kavita R, Veena B, Kagdiyal V, et al. Estimation of Mineral Oil Contamination in Synthetic Lubricants by 1H NMR Spectroscopy. Anal Chem Ind J. 2016;16(14):103.
Abstract
One of the major phenomena occurs during the field trial of synthetic lubricants (SL) is their contamination with mineral oils due to leaking, improper handling and lubrication practices. Effect of this contamination beyond certain percentage generates excess foam, deteriorates air release, clogs the filters etc. which finally leads to damage of the equipment. Hence, estimation of mineral oil in a synthetic lubricant would be of great significance for quality monitoring and maintaining consistent performance. A 1H NMR based method for the estimation of mineral oil in SL based on group-V base oils has been developed. SL includes synthetic esters, phosphate esters, polysiloxane based oil etc. generally used as fire resistant hydraulic fluids, compressor oil, aviation lubricants, refrigeration oils etc. The quantitative estimation of the percentage age of mineral oil contamination has been carried out by unambiguous assignment of characteristic protons of SL over mineral oil using suitable internal standards. In the present study physical blends of pure synthetic esters and finished SL with mineral oil (Gr-II & III) have been studied followed by actual field trial samples. The new method also estimates mineral oil contamination in SL made of mixture of polyalphaolefin (PAO, group-IV) and group-V oils. The method has been validated by known blends. The method is highly repeatable and efficient over 5% (w/w) minreal oil contamination.
Keywords
1H NMR; Contamination; Synthetic fluid; Field trials
Introduction
Synthetic Lubricants (SL) are composed of API group-IV and/or -V base oils and tailor made additives. The demand for SL in the field of lubrication is growing steadily over the years on account of superior performance than those found in traditional mineral oil based lubricants, especially under severe operating temperatures and pressures conditions [1]. Jet engines, for example, require the use of synthetic oils (SO), whereas aircraft piston engines do not [2]. Moreover, SL are generally environmentally benign and better aligned with government’s policies. Unlike mineral base oils, which are complex mixtures of naturally occurring hydrocarbons (paraffins, aromatics, naphthenes, polars), synthetic base stocks are man-made and tailored to have a controlled molecular structure with predictable properties. One of the major phenomena occurs during the field trial of SL is their contamination with traditional mineral oils mainly due to leaking, improper handling and lubrication practices. Effects of this contamination beyond a certain percentage include excess foam generation, poor air release, filter clogging, malfunctioning and finally damage of the equipment’s [3,4]. In our long experience of field trials and literature study [4], barring from specialized applications such as turbine governor fluid, the mineral oil contamination less than 5% does not associate with any significant deterioration of performance. The combination of oil analysis and effective deployment of lubrication management as well as machine maintenance would enable the reliability and in turn would deliver real benefit to the process engineer. Hence, the estimation of mineral oil as contaminant from field trial of SL has been of great importance. There has been a felt need to develop a practical, simple and rapid protocol to identify and quantify the mineral oil contamination in SL, which would thus help in taking corrective measures at right time and reduces the maintenance costs.
Mineral oil and PAO based hydraulic fluid as contaminant in turbine engine oil made of polyol ester have been identified and quantitatively estimated by GC-CIMS technique. In their communication, Webster et al. [5] have demonstrated that the difficulties arising out of similar retention characteristics in GC and similar fragmentation patterns in electron impact (EI) m/z for both hydraulic oils and polyol esters could successfully be overcome by using softer mass fragmentation protocol of chemical ionization technique. Contamination of mineral oil in animal feed and food products has also been studied by Grob et al. using LC-GC-FID [6]. However these methods are developed for specific application and lack generality in addition to be more time consuming. To the best of our knowledge there has not been any report of estimation of mineral oil in SL by using a rapid method like 1H NMR technique. NMR spectroscopic technique has long been used for both qualitative [7,8] and quantitative [9,10] studies of petroleum products. Moreover there have been a significant number of studies on SO and SL by NMR spectroscopy [11-20]. The main focus of these studies revolves around to understand the qualitative composition, structural characterization and relation of chemical composition with physical properties. Thermal degradation process of SLs and thermal stability of synthetic and semi-synthetic engine oils have recently been studied by Santos et al. [21] and Tripathy et al. [22] respectively.
Herein, we report a rapid and simple 1H NMR spectroscopic method for the estimation of mineral oil in SL of group-V base oils. The method uses either Hexamethyldisiloxane, HMDSO or Dioxane (depending on the nature of SO) as the internal quantitative reference standard with respect to which the percentage age of SOs, such as polyol ester, phosphate ester and polysiloxane based oils, has been estimated. The difference between the weight taken and SO estimated concomitantly provided the percentage age of mineral oil in the sample.
Experimental
Sample
Following laboratory prepared blends of phosphate ester, pentaerythritol ester, polydimethyl siloxane and composite SL of ester and PAO (blends 1-6, 7-12, 13-16 & 17-20 respectively) (Table 1) have been analyzed by 1H NMR while HMDSO was used as the internal reference standard except in case of siloxane, for which dioxane was used as the internal standard. Three different solutions of a tri-aryl phosphates comprising of cresol, ethylphenol and xylenol in known composition and four finished SL based on phosphate esters and polyol esters (two each) have also been analyzed in order to verify the accuracy of HMDSO-based approach and study the effect of additive in the estimation respectively (vide infra). Several field trail samples based on phosphate esters, pentaerythritol esters, adipic ester have also been studied with variable concentration of samples and HMDSO. HMDSO and dioxane were purchased from Sigma-Aldrich Chemical Company, New Delhi. SOs, finished SLs and actual contaminated samples were collected from different field trial locations in India.
| Details (wt%) | Blend 1 | B-2 | B-3 | B-4 | B-5 | B-6 |
|---|---|---|---|---|---|---|
| Phosp. Esters | 96.1 | 91.4 | 84.2 | 81.7 | 59.8 | 28.9 |
| Mineral Oil | 3.9 | 8.6 | 15.8 | 18.3 | 40.2 | 71.1 |
| Blend 7 | B-8 | B-9 | B-10 | B-11 | B-12 | |
| Polyol Esters | 97.2 | 94.1 | 88.6 | 81.9 | 72.7 | 58.5 |
| Mineral Oil | 2.8 | 5.9 | 11.4 | 18.1 | 27.3 | 41.5 |
| Blend 13 | B-14 | B-15 | B-16 | |||
| Silicon Oil | 94.4 | 79.9 | 69.5 | 60.6 | ||
| Mineral Oil | 5.6 | 20.1 | 30.5 | 39.4 | ||
| Blend 17 | B-18 | B-19 | B-20 | |||
| Ester-PAO | 100 (20:80) | 90.7 | 78.4 | 48.1 | ||
| Mineral Oil | 0 | 9.3 | 21.6 | 52.9 |
Table 1. Various blends of SL analysed
NMR method
All proton NMR spectra were recorded on a Jeol ECA-500 NMR spectrometer operating at the proton frequency of 500 MHz, spectral width 7512 Hz (-2.5 ppm to 12.5 ppm), 90° pulse=10.7 μs, relaxation delay=20 s, digital resolution: 0.57 Hz/point. 16 repetitions were averaged with 32 K data point and 6.38 min experimental time. All the NMR spectra were integrated after baseline correction, and a mean of minimum three integration values has been taken for each calculation. The sample preparation method has been described in the supporting information.
Selection of a reference compound and fixing the recycle delay (d1)
Recently it has been demonstrated [23] that hexamethyldisiloxane, HMDSO is an excellent internal quantitative reference standard, meeting all the desirable characteristics for NMR purposes. Interestingly dioxane can also be used as internal quantitative reference standard for silicon based oil, where chemical shift region overlaps with that of HMDSO. Purity of HMDSO and dioxane used has been thoroughly checked and discussed in our previous publication [23]. It has been found that increasing the relaxation delay from 5s to 20s does significantly influences the integral value and so most of the samples were recorded with 20 s relaxation delay.
Results and Discussion
Principle of the estimation
The philosophy of the estimation of mineral oil contamination in synthetic fluids has been explained by studying two different cases as described below. The methodology lies (i) on unambiguous assignment of a characteristic resonance signal of at least one set of protons of the synthetic fluid workably unmerged with mineral oil, (ii) on the determination of average molecular weight and average proton contribution in case of multicomponent system with overlapped proton resonance and (iii) on complete elucidation of the structure of the synthetic fluid.
Case 1: For a pure single component SL
The amount (or %) of mineral oil (WOil) in a blend (Wblend) (or a contaminated sample) of single component SO (Wx) with mineral oil has been estimated by quantitative 1H NMR experimentation while compared with known amount of HMDSO (WHMDSO) or known amount of dioxane (WDO). The following equations have been used for the estimation.
Wx=(WHMDSO × Mx × NHMDSO × Ix)/(MHMDSO × Nx × IHMDSO)=0.11085 × (WHMDSO × Mx × Ix))/(Nx × IHMDSO) (1)
Where, Wx=Weight of component to be estimated, WHMDSO=Weight of HMDSO taken, Mx=Molecular weight of component, Ix=Integral value of the relevant chemical shift region of the component, Nx=Number of protons in the chemical shift region with integral Ix for the component, IHMDSO=Integration value for HMDSO at 0.07, NHMDSO=18 and MHMDSO=162.38.
Wx=(WDO × Mx × NDO × Ix)/(MDO × Nx × IDO)=0.090796 × (WDO × Mx × Ix))/(Nx × IDO) (1a)
Where, Wx=Weight of component to be estimated, WDO=Weight of dioxane taken, Mx =Molecular weight of component, IDO =Integration value for dioxane at 3.65, NDO=8 and MDO=88.11.
The percentage of base oil in the sample or in the blend could then be estimated using Eq. 2 and Eq. 3 as follows:
WOil=Wblend-Wx (2)
Mineral Base Oil (%)=(WOil/ Wblend) × 100 (3)
Tricresyl phosphates, in regioisomeric mixture (p- & m-), have been blended with mineral base oil (Group-II) in different percentages and was then estimated using dioxane as reference standard following the general principle described above (see supporting information).
Case 2: For multicomponent SL
For multicomponent SO, at least one reasonably good resolvable chemical shift region for each component, the above method has been extended and has been demonstrated by a physical blend of tri-cresyl phosphates (WTCP), tri-xylyl phosphates (WTXP) and tri-ethylphenyl phosphates (WTEPP) in group-II mineral oil. In the first step, by considering the integral values separately for each component between δ 2.0 to 2.7 the amount of three different phosphates in an unknown mixture has been estimated by quantitative 1H NMR experiment using Eq. 1. In the second step, the % of mineral oil has been estimated as follows:
WOil=Wblend-(WTCP+WTXP+WTEPP) (4)
Or, by considering the integral values together for all the components in the aromatic region between δ 6.5 to 7.5 ppm, the amount of TCP, TXP and TEPP (WSO≡WTCP+WTXP+WTEPP) can be estimated (see supporting information).
A systematic study on a complex mixture of tri-aryl phosphates with Gr-III base oil has been carried out using HMDSO as the reference standard and the results are summarized in Table 2.
Phosphate esters
Phosphate esters, especially triaryl-phosphates (TAPs), the most widely used fire resistant hydraulic fluid, are occurred as a complex isomeric mixture of various possible phosphates, e.g., tri-cresyl phosphates (TCP), tri-xylyl phosphates (TXP), tri-ethylphenyl phosphates (TEPP), tri-t-butylphenyl phosphate (TTBPP), tri-i-propylphenyl phosphate (TIPPP) tri-phenyl phosphate (TPP) etc. and their compound isomers. Contamination of these synthetic fluids by mineral oil is a common occurrence, especially during field trials.
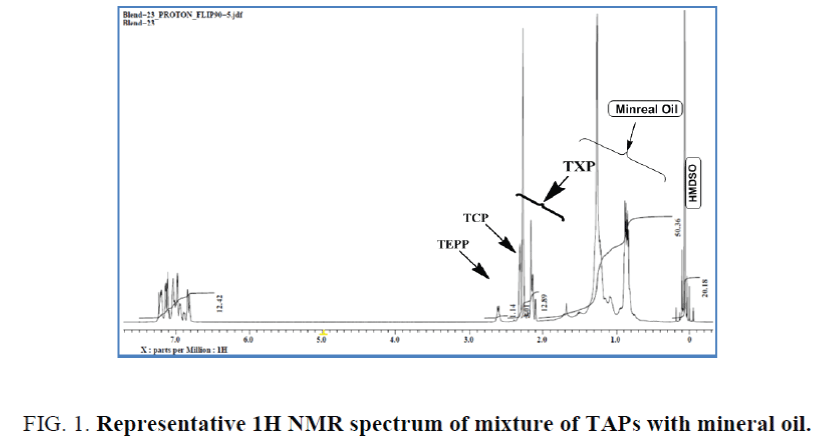
A mixture of complex phosphate esters: A mixture of tri-aryl phosphate esters in which the aryl groups are contributed by cresyl-, xylenyl- and ethylphenyl- in the ratio of ~25%, ~60% and ~15% respectively was chosen for the study. The weight ratio of different constituent phenols was estimated by the integrals of methyl (cresyl & xylenyl) and methylene (ethylphenyl) signals. The known percentage age of phenols was verified by quantitative 1H NMR and the accepTable integral regions by which the optimum values were achieved have been established. While the methyl protons of the cresyl- and xylenyl- moieties appeared at δ 2.3-2.4 and δ 2.08-2.3 ppm respectively, the methylene protons of the ethylphenyl moiety comes at δ 2.63 ppm (Figure 1). These assignments were done by 1H, 13C and 2D NMR and corroborated by literature study [24]. The same integral values were used for the estimation by way of comparing with HMDSO. The results are summarized in Table 2. It has been noticed from Table 2 that the estimated persentage age of cresyl-, xylenyl- and ethylphenyl- groups were somewhat comparable with the actual values.
Figure 1. Representative 1H NMR spectrum of mixture of TAPs with mineral oil.
| No. of Entry | wt% of Phenol (Independent of HMDSO) | wt% of Phenol (by HMDSO) | Error* | ||||
|---|---|---|---|---|---|---|---|
| Cresyl | Xylyl | Ethylphenyl | Cresyl | Xylyl | Ethylphenyl | ||
| Sol 1 | 25.5 | 59.8 | 14.7 | 24.3 | 58.1 | 14.4 | 3.2 |
| Sol 2 | 25.3 | 59.6 | 15.1 | 24.0 | 57.5 | 14.1 | 4.4 |
| Sol 3 | 24.4 | 61.1 | 14.5 | 23.3 | 57.2 | 14.0 | 5.5 |
*Deviation from 100%; contributed also by presence of impurity in the sample.
Table 2. Verification of the composition of aryl groups in triaryl phosphates.
However, while compared with known amount of HMDSO the total percentage age of these three components estimated was accounted for only ~95% to 97% of the weight taken, indicating presence of other component(s) in the sample. The most dilute samples with respect to triaryl-phosphates gave most erroneous values (Solution 3, The sample was then contaminated with mineral base oil in different ratios. The percentage age of mineral oil was then estimated by quantitative 1H NMR using equations 1, 3 and 4, in which primarily the aliphatic protons of the triaryl phosphate have been considered for the estimation. The result was further corroborated while estimated by considering aromatic protons, average proton contribution, average molecular weight of the triaryl phosphate using Equation 3. Both the results have been tabulated in Table 3. The estimation shows excellent correlations between two different approaches. One example of such estimation has been shown with the NMR spectrum in Figure 1. for which the mineral oil has been estimated to be 39% by aliphatic protons and 40.1% by aromatic protons against 40.2% blended (case 5, Table 3). It is clearly evident from Table 3 that mineral oil contamination less than 5% gave erroneous result, in terms of relative percentage age w.r.t. mineral oil weighted, even after several repetitions. In order to examine the effect of additives in the estimation two finished SL with additives have been quantitatively analysed by known amount of HMDSO. It was found that whether it is pure SO or finished SL does not make any difference in the estimation of contamination until the percentage age of additives remains ~1% to 2%. Several field trial samples of phosphate esters have been analyzed for mineral oil contamination (see supporting information).
| S. No. | Min oil Wtd. (%) | Min oil estd. (%) (by CH3- protons) |
Error*(%) | Min oil estd. (%) (by Ar-protons) |
Error* (%) |
|---|---|---|---|---|---|
| 1 | 3.9 | 2.7 | 30.8 | 2.9 | 25.6 |
| 2 | 8.6 | 7.9 | 8.1 | 8.4 | 2.3 |
| 3 | 15.8 | 15.3 | 1.9 | 15.6 | 1.3 |
| 4 | 18.3 | 17.4 | 4.8 | 18.2 | 0.5 |
| 5 | 40.2 | 39.0 | 3.3 | 40.1 | 0.3 |
| 6 | 71.1 | 68.4 | 3.8 | 69.5 | 2.3 |
*Errors indicate % age errors w.r.t. mineral oil weighted.
Table 3. Mineral oil in a three component phosphate ester system using HMDSO.
Polyol esters
Synthetic polyol esters (Figure 2.) have been used successfully in lubrication for more than 60 years and are the preferred stock in many severe applications. For example, esters have been used exclusively in jet engine lubricants worldwide for over 50 years due to their unique combination of low temperature flow ability with clean high temperature operation. Esters are also the preferred stock in the new synthetic refrigeration lubricants used with CFC replacement refrigerants. In automotive applications, the first qualified synthetic crankcase motor oils were based entirely on ester formulations and these products were quite successful when properly formulated.
In lubricant formulation pentaerytritol esters (PE), such as PE esters of oleate, linolate, 2-ethyl hexanoate etc. are widely used as fire resistant hydraulic oil. During the field trials these formulations get exposed to contamination, mostly by mineral oil. Thus PE ester of 2-ethyl hexanoic acid has been chosen for our study. Several blends have been prepared and studied, the results of which are summarized in Table 3. Again it was found that contamination less than 5% provides erroneous result in its relative percentage age w.r.t. percentage age of mineral oil weighted not in absolute terms. For examples, in absolute terms 3.9% weighted mineral oil has been estimated to be 2.9% (Blend 1, Table 3) and 2.8% to 1.8% (Blend 7, Table 4) but those in relative percentage age of errors w.r.t. percentage age weighted have been converted to 25.6% and 35.7% respectively. Effect of additives in the estimation of mineral oil in PE ester based SL has also been studied. Like previous case it was observed that with <2% additive the estimation provided excellent accuracy while estimated by HMDSO.
| Blend No. | Min Oil Weighed (%) | Min Oil Estimated (%) | Error(%) |
|---|---|---|---|
| 7 | 2.8 | 1.8 | 35.7 |
| 8 | 5.9 | 5.2 | 11.9 |
| 9 | 11.4 | 11.3 | 0.9 |
| 10 | 18.1 | 17.8 | 1.6 |
| 11 | 27.3 | 26.9 | 1.5 |
| 12 | 41.5 | 40.7 | 1.9 |
Table 4. Mineral oil (Gr-III) in PE ester using HMDSO.
Field trial samples: Several synthetic ester samples based on PE ester of, primarily, oleic acid, used as fire resistant hydraulic fluid (FRHF), have been analyzed for mineral oil contamination using the developed method and some of them have been summarized in Table 5. It was found that the contamination increases almost linearly with the duration of field trial indicating possible contamination during topping up of FRHO. As evident by Table 5 as well as analysis of more filed trial samples based on phosphate esters (see supporting information), it can be argued that the samples were analyzed only after the contamination reaches more than 5 wt%.
| Sample | Time in field trial (h) | Mineral oil (%) |
|---|---|---|
| FRHF-1 | 886 | 28.0 |
| FRHF-2 | 704 | 27.6 |
| FRHF-3 | 700 | 21.5 |
| FRHF-4 | 690 | 19.8 |
| FRHF-5 | 680 | 20.9 |
| FRHF-6 | 412 | 12.8 |
| FRHF-7 | 330 | 5.6 |
| FRHF-8 | 212 | ~ |
Table 5. Mineral oil contamination during field trial of FRHO.
Silicon based oil: polydimethyl siloxane/polydiethyl siloxane
Silicon based base oils such as polydimethyl siloxane, polydiethyl siloxane, polydimethyl-poly(methylphenyl) siloxane etc. (Figure 3.) have been widely used as synthetic base oil of special use as well as high viscosity fluid for industrial applications. Contamination of mineral base oil could significantly damage the performance of these special base oils. As the silicon base oil has chemical shift region which could overlap the signal arises out of HMDSO, dioxane would be the viable alternative as reference standard. Instrument oil made of polydimethyl siloxane is blended with Gr-II base oil for the study and the results are summarized in Table 6.
| Blend No. | Min Oil Weighed (%) | Min Oil Estimated (%) | Error (%) |
|---|---|---|---|
| 13 | 39.4 | 38.7 | 1.8 |
| 14 | 30.5 | 29.7 | 2.6 |
| 15 | 20.1 | 19.5 | 3.0 |
| 16 | 5.6 | 4.4 | 21. 4 |
Table 6. Mineral oil in a polydimethyl siloxane based instrument oil.
The result below clearly indicates that though the estimation of base oil considered the unit molecular weight of the silicon oil as 74.15, not the actual molecular weight of the polymer, did not affect the estimation. Rather the error seems to be significantly lower over a long range of base oil contamination.
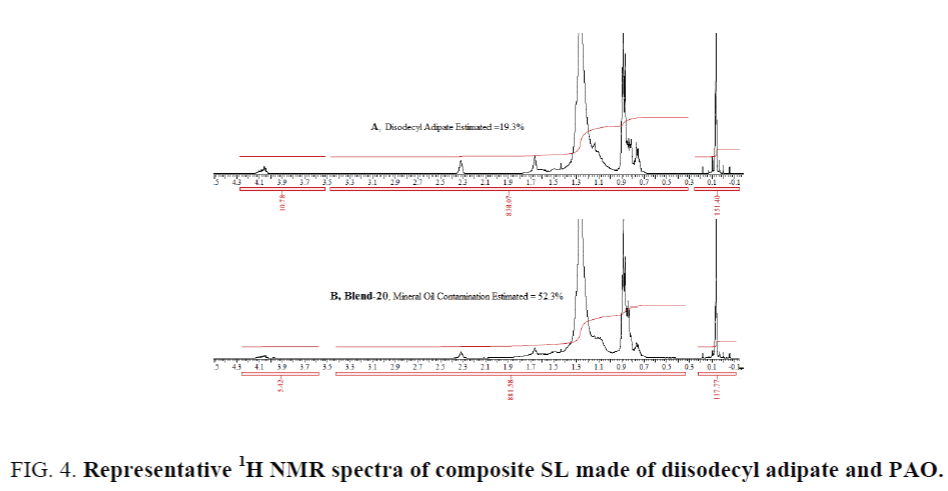
A case study of composite synthetic compressor oil: A blend of diisodecyl adipate and poly alpha olefin (PAO): Some SLs are not made of only group-V esters or group-IV PAO exclusively, rather made of a suitable combination these two. In such SL, if the percentage age and structure of group-V synthetic ester is determined (or known), the contamination of mineral oil can be estimated irrespective of the nature of PAO. A composite SL made of 20% diisodecyl adipate and 80% PAO, a product under trial, has been studied. As requirement of estimating the mineral oil contamination in the field trial sample (a composite SL made of 20% diisodecyl adipate and 80% PAO) arises, three lab-made blends along with several used samples from field trial have been analyzed for mineral oil contamination. The results for lab made blends are shown in Table 7. The percentage age of diisodecyl adipate have been estimated to be 19.4% vs 20% originally blended. Following the described method the amount of diisodecyl adipate in the blends (18, 19 and 20) and in the field trial samples have been estimated. Using the ratio in the fresh sample (19.4:80.6 as estimated) amount of PAO was calculated. Deduction of the sum of diisodecyl adipate and PAO from the known amount weighted sample finally provides the mineral oil contamination. An example of 1H NMR spectra have been shown in Figure 4, in which spectrum-A represents a case of fresh composite SL estimating 19.3% diisodecyl adipate and spectrum-B is for blend 20 (Table 7) that estimates mineral oil contamination 52.3%.
| Blend No. | Min Oil Weighed (%) | Min Oil Estimated (%) | Error(%) |
|---|---|---|---|
| 17 | 0.0* | 0.0* | - |
| 18 | 9.3 | 8.9 | 4.3 |
| 19 | 21.8 | 21.1 | 3.2 |
| 20 | 52.9 | 52.2 | 1.3 |
*In blend 17 the percentage age of diisodecyl adipate has been estimated to be 19.4% vs. 20% actual.
Table 7. Mineral oil in composite SL made of PAO and adipate ester.
Figure 4. Representative 1H NMR spectra of composite SL made of diisodecyl adipate and PAO.
Repeatability and reproducibility in the estimation of mineral oil
The accuracy of this 1H NMR method is heavily dependent on the accuracy of the weight taken for reference compound as well as for the sample chosen along with accurate assignment of the representative signals for SL. The mineral oil contamination for few lab blends (blends 3-6 of TAPs) and some of the field trial samples (FRHO 1-4 of PE ester of oleic acid) have been estimated three times with variable amount of HMDSO and samples under same experimental condition. The results are summarized in supporting information. It has been found that for lab blend phosphate ester samples the variations in the repetitions is more (RSD=0.29) than that of oleyl ester of pentaerythritol field trial samples (RSD=0.12). Considering the fact that the phosphate esters are of a complex mixture of different TAPs and oleyl ester is relatively simple the relative standard deviations are well accounted.
The reproducibility of the developed proton NMR method has been established and found to be satisfactory when some of the samples were recorded by two different operators following same experimental procedure.
Conclusions
A simple 1H NMR spectroscopic method has been developed for the estimation of mineral oil contamination in various kinds of synthetic fluids. The method is applicable for the estimation of mineral oil in a wide range of synthetic lubricants made of phosphate esters, PE esters, polydimethyl siloxanes, TMP/TME esters, adipate esters, composite mixture of group-IV and group-V base oils etc. Hexamethyldisiloxane, HMDSO or dioxane as internal standards have been used for quantitative estimation of mineral oil contamination, alone serves as a frequency as well as quantitative reference for proton NMR. The method works excellent for relevant samples of more than 5% (w/w) mineral oil contamination. The method is surely not sensitive for estimating 1% to 2% mineral oil contamination owing to the fact that the SL generally are not very pure compounds and NMR itself is not a highly sensitive technique. This method, however, offers a rapid, highly repeaTable and efficient protocol to the analytical chemists to monitor performance of SL during field trials via estimation of mineral oil contamination and provide space to avoid cumbersome methods where peak assignment itself could be a difficult task for unknown contaminants. The method can also be applied to estimate the percentage age of synthetic oil in semi-synthetic oils.
Acknowledgements
The authors wish to acknowledge the management of IOCL R&D Centre for proving necessary facilities to carry out the work and granting permission to publish this paper.
Supporting information
Includes sample preparation details, Tables 1S-5S, details of real sample analyzed and Figures 1S-4S, few relevant spectra.
References
- Stipanovic AJ. Hydrocarbon Based Oil Chemistry. In: Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing.Pennsylvania: ASTM International. 2003; 169-84.
- Wills JG. Lubrication Fundamentals. Mobil Oil Corporation.New York: 1980.
- Theissen HW. Effects of Contamination of Biobased Hydraulic Fluids with Mineral Oil. JASTM Int. 2009;6(1):1-9.
- Golumb S, Robin L. Synthetic Lubricants, Contamination Control and Oil Analysis Team Up. PractOil Analys. 1999;5:1-3.
- Webster RL, Evans DJ, Rawson PM. A method for the identification and quantitation of hydraulic fluid contamination of turbine engine oils by gas chromatography–chemical ionisation mass spectrometry. LubricatSci. 2012;24(8):373-81.
- Grob K, Vass M, Biedermann M, et al. Contamination of animal feed and food from animal origin with mineral oil hydrocarbons. Food Addit Contam. 2001;18(1):1-10.
- Gillet S, Delpuech JJ, Valentin P, et al. Optimum conditions for crude oil and petroleum product analysis by carbon-13 nuclear magnetic resonance spectrometry. Anal Chem. 1980;52(6):813-7.
- Kvalheim OM, Aksnes DW, Brekke T, et al. Crude oil characterization and correlation by principal component analysis of carbon-13 nuclear magnetic resonance spectra. Anal Chem. 1985;57(14):2858-64.
- Bansal V, Kapur GS, Sarpal AS, et al. Estimation of Total Aromatics and Their Distribution as Mono and Global Di-Plus Aromatics in Diesel-Range Products by NMR Spectroscopy. Energy Fuels. 1998;12(6):1223-7.
- Bansal V, Krishna GJ, Chopra A, et al. Detailed Hydrocarbon Characterization of RFCC Feed Stocks by NMR Spectroscopic Techniques. Energy Fuels. 2007;21(2):1024-29.
- Sarpal AS, Kapur GS, Mukherjee S, et al.Characterization by 13C NMR spectroscopy of base oils produced by different processes. Fuel. 1997;76:931-7.
- Sarpal AS, Sastry MIS, Bansal V, et al. Correlation of structure and properties of groups I to III base oils. LubricatSci. 2012;24(5):199-215.
- Kapur GS, Sarpal AS, Jain SK, et al. Detailed characterisation of heavy alkylated benzene fluids by multipulse one- and two-dimensional NMR spectroscopy. JSynthLubricat. 2000;17(1):41-54.
- Kapur GS, Sarpal AS, Bhatnagar AK.Temperature-dependent effects in base oils: Carbon-13 NMR spin-lattice relaxation time and viscometry studies. LubricatSci. 2002;14(3):287-302.
- Sarpal AS, Sastry MIS, Kapur GS, et al. Molecular dynamics studies of a lubricant system by NMR spectroscopic techniques. LubricatSci. 2004;16(4):361-75.
- Sarpal AS, Bansal V, Sastry MIS, et al. Molecular spectroscopic studies of the effect of base oils on additive-additive interactions. LubricatSci. 2003;16(1):29-45.
- Sarpal AS, Christopher J, Mukherjee S, et al. Study of additive-additive interactions in a lubricant system by NMR, ESCA, and thermal techniques. LubricatSci. 2005;17(3):319-45.
- Kapur GS, Sarpal AS, Sarin R, et al. Detailed characterisation of polyalphaolefins and their branched structures using multi-pulse NMR techniques. JSynthLubricat. 1998;15(3):177-91.
- Sarpal AS, Kapur GS, Bansal V, et al.Direct Estimation of Aromatic Carbon (Ca) Content of Base Oils by (1)H-Nmr Spectroscopy. PetrolSciTechnol. 1998;16(7-8):851-68.
- Sarpal AS, Sastry MIS, Kumar R, et al. Molecular Dynamics of Synthetic-Based Lubricant System by Spectroscopic Techniques-Part 1. TribolTransact. 2013;56(3):442-52.
- Santos JCO, Santos IMG, Souza AG. Thermal Degradation Process of Synthetic Lubricating Oils: Part I—Spectroscopic Study. PetrolSciTechnol. 2015;33:1238-45.
- Tripathi AK, Vinu R. Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils. Lubricants. 2015;3(1):54-79.
- Mondal S, Kumar R, Bansal V, et al. A 1H NMR method for the estimation of hydrogen content for all petroleum products. JAnalytSciTechnol. 2015;6:24-33.
- Buchanan GW, Wightman RH.Malaiyandi M. A carbon-13 nuclear magnetic resonance spectral investigation of substituted triphenyl phosphates. OrgMagnetResonance. 1982;19(2):98-101.