Original Article
, Volume: 17( 2)Escherichia coli Detection Using Glycine Coated Cobalt Ferrite Nanoparticles
- *Correspondence:
- Viswanathan K , Translational Research Platform for Veterinary Biologicals, Tamil Nadu Veterinary and Animal Sciences University, Madhavaram Milk Colony, Chennai-600 051, Tamil Nadu, India
Tel: +0918754960627; E-mail: viswanthanphd@yahoo.com
Received: September 12, 2017; Accepted: September 14, 2017; Published: September 22, 2017
Citation:Viswanathan K, Chitrapriya K. Escherichia coli Detection Using Glycine Coated Cobalt Ferrite Nanoparticles. Anal Chem Ind J. 2017;17(2):121
Abstract
In this report, the E. coli detection method was developed by using glycine coated cobalt ferrite nanoparticles (Gly/CoFe2O4 ) as a collection probe from the drinking water sample. The Gly/CoFe2O4 nanoparticles were used to capture and separate E. coli from the drinking water. The detection is based on beta-glucuronidase enzyme present on the bacteria lysate. The enzyme activity was measured using 4-methylumbelliferyl β-D-glucuronide hydrate as fluorogenic substrates. The calibration curve of the E. coli showed the linear range to be between 1 × 102 ~ 1 × 104 CFU/ml (R2 =0.9944). The minimum detectable concentration was 1 × 102 CFU/mL. The developed new method offered the best way to determine E. coli from the water sample with a total analysis time of 45 min.
Keywords
Cobalt ferrite nanoparticle; Magnetic capture; Glycine; Bacteria
Introduction
Water contamination is the major problem in developing countries, and especially by the bacteria. Three different bacteria routinely monitored by water bodies such as faecal indicators bacteria (FIB), E. coli and enterococci [1-3]. The E. coli detection mainly relies on cultivation of the target organism which requires relatively long incubation periods (up to 48 h) and this poses an inconvenience. Previously PCR based amplifications, nucleic acid based detections, ELISA based immune assay and enzyme substrate based detection methods are already developed by the researchers. The PCR and nucleic acid based detections are not yet routinely used due to the fact that they are not sensitive enough, the equipment is expensive, they are strain specific, or they detect total rather than viable cells. Furthermore, ELISA method requires extensive sample preparation steps and is not yet suitable for rapid on-site monitoring [4-6]. The combination of nanotechnology with the analytical method helps to improve the sensitivity of the assay, reduce the running cost and time [7,8]. Magnetic nanoparticles are widely applied for the specific enrichment of target cells from the complex matrix, due to their strong external magnetic field response [9,10]. The surface modification of nanoparticles adds to their usefulness and alters their intrinsic characteristics. The assemblies of biomolecules such as antibodies, enzymes, and streptavidin on the surface of nanoparticles provide the platform for bioanalysis [11-14]. In the water industries specific enzyme biomarker (β-galactosidase, β-glucuronidase and β-glucosidase) based bacteria detection were widely used, and for the pre concentration and recovery of bacteria from the sample they used syringe filters or other enrichment techniques. So in this work we developed glycine coated CoFe2O4 nanoparticles as an enrichment nano probe to collect up to 92% of E. coli within 20 min. The enriched bacteria were lysed with lysis buffer and the β-glucuronidase concentration was detected using 4-methylumbelliferyl β-D-glucuronide hydrate as substrate. The major advantage, this method does not required any recovery and pre concentration steps, and the assay within 45 min and highly suitable for on-site monitoring.
Materials and Methods
Glycine coated CoFe2O4 nanoparticles
Glycine coated CoFe2O4 nano particles were synthesized based on co-precipitation method. Briefly, 0.54 g of iron (ii) nitrate monohydrate, 0.234 g of cobalt (ii) nitrate hex hydrate was dissolved in 20 mL of milli-Q-water, and then 20 mL of ammonia was added, and the sample was heated at 80°C for 30 min with stirring. Finally 25 mL of 2.5 g of glycine was added and the solution was heated another 90 min. finally the particles were collected by centrifugation at 8000 rpm for 10 min. The synthesized glycine coated CoFe2O4 particles were collected by magnetic separations and washed repeatedly. For the unmodified particle synthesis, after adding ammonium hydroxide the final solution was heated at 80°C for 30 min. Both the synthesized particles were dried at 55°C and stored at room temperature before use. For the quantification of glycine functional groups on the nanoparticle surface was carried out by ninhydrin colorimetric assay [15,16]. The ninhydrin reagent (500 μL of 0.2% w/v in 0.1 M buffer phosphate, pH 9) was added to 200 μL of nanoparticle sample and the mixture heated in a boiling water bath for 15 min. Samples were cooled to room temperature and placed on a magnetic separator to remove the nanoparticles. The absorbance of supernatants was measured at 570 nm on a microplate and the amine content quantified using glycine standard curves.
Magnetic collection of bacteria
To study the collection efficiency of the bacteria, 5 × 106 CFU/mL of E. coli were taken and the dilution was confirmed both plate counting (no. of colonies × dilution factor)/volume inoculated=CFU/ml) and UV absorption at 600 nm. Following addition of the Gly coated CoFe2O4 nanoparticles (to each of the above bacterial isolates) the samples were incubated at 37°C for 20 min and the bound bacteria was captured using a magnet to determine the capture efficacy based on plate counting, optical density at 600 nm, and it also confirmed by using TEM.
For the estimation of lower capture efficiency, the E. coli was serially diluted up to 1 × 101 CFU/mL. To each of the above serial dilutions, 25 mg/mL of Gly coated CoFe2O4 nanoparticles were mixed and incubated for 20 min at 37°C. The number of bound bacteria (capture efficacy) was determined in the supernatant by plating the supernatant in the selective agar and the optical detection at 600 nm.
Quantification of bacteria in samples
The bacteria detection involved three major steps such as:
1. Glycine coated CoFe2O4 nano particles reacted with bacteria, magnetic separations and followed by washing with PBS buffer.
2. Addition of lysis buffer with bacteria captured glycine conjugated CoFe2O4 particles.
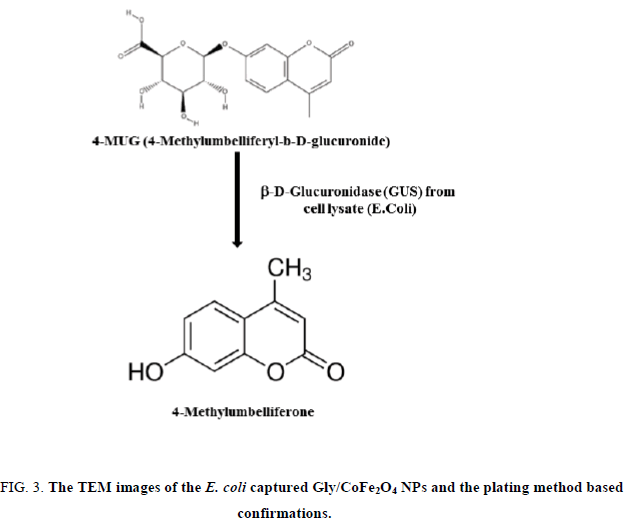
3. Fluorescence measurement. Briefly, 100μL of 25 mg/mL glycine conjugated CoFe2O4 particles, 100μL of serially diluted bacteria samples, and the concentration ranging from 1 × 101~ 1 × 106CFU/mL were added into 1 mL eppendrof tube. The tube was agitated for 10 min (hand agitation) and kept under magnetic separators, the unbound bacteria were removed and the bacteria bound nanoparticles were lysed with lytic reagent (2 mL) and vigorously mixed for 5 min and followed by magnetic separation. The separated lysate was mixed with 50μL of 4-methylumbelliferyl β-D-glucuronide hydrate (100 mm in DMSO) and the fluorescent was read at 445 nm (excitation wave length 365 nm).
Results and Discussion
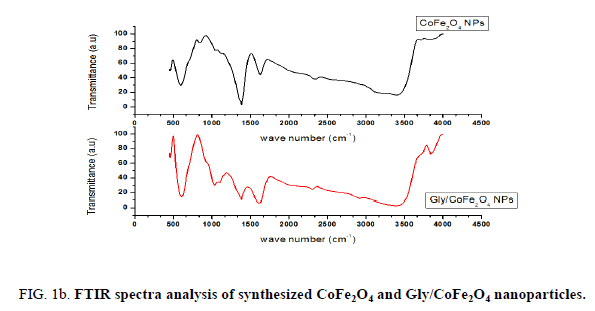
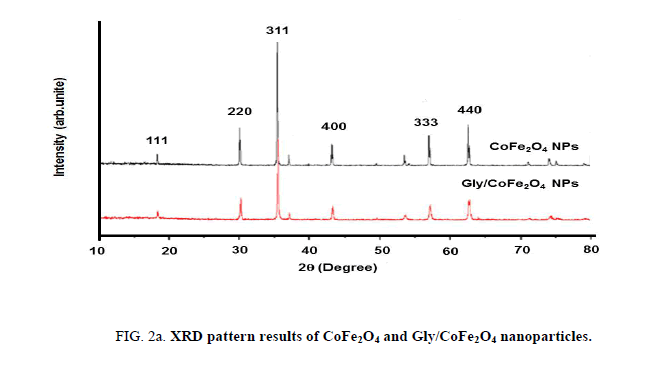
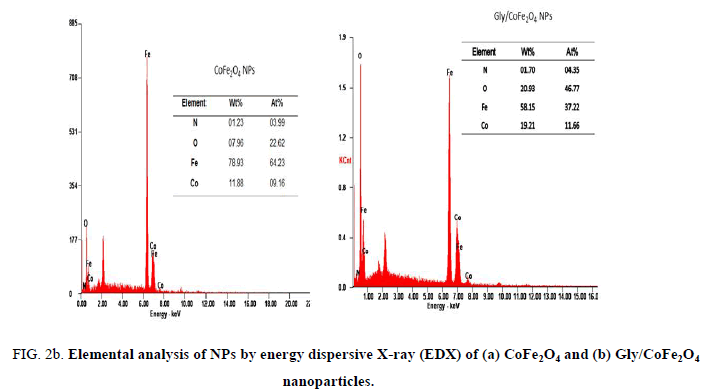
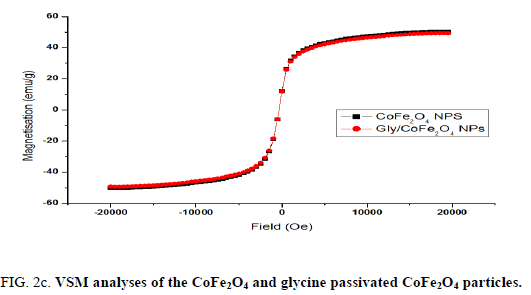
Nanoparticles were synthesized based on co precipitation process using iron (ii) nitrate monohydrate and cobalt (ii) nitrate hexahydrate. The reaction between iron (ii) nitrate and cobalt (ii) nitrate ions were catalyzed by using ammonium hydroxide. For the glycine surface modifications, the glycine was added during synthesis and it offered the NH2 functional group on the surface. The size was confirmed by using SEM and it indicated that the size was around 10nm to15 nm (Figure. 1a). The glycine surface modification was confirmed by using FTIR and the result was shown in Figure. 1b. Based on the results the broad bands of 3394-1 and 2916 cm-1identified related with γ (OH),1383 cm-1(δ CH2-COO- vs. COO-group of glycine), 1105 cm-1 and 1031 cm-1 attribute to NH3+ also recorded similar like previous publications16. The vFe-O peak was recorded at 587 cm-1 and the glycine peak was recorded at 1616 cm-1. For the comparison we also scanned CoFe3O4 particles and it showed broad bands of 3394 cm-1 and 2343 cm-1 that were related to the γ (OH), 1300 cm-1 (COO-group), 596 cm-1 attributed to vFe-O, and 1629 cm-1 indicated presence of NH moiety. The X-ray diffraction (XRD) patterns of the nanoparticles were compared with standard JCPDS indexed patterns and the reference card number is: 22-1086 based on that the peak corresponding to the planes (3 1 1), (4 4 0) and (2 2 0) confirms the phase formation of pure CoFe2O4 with a well-defined spinel structure. The glycine modified CoFe2O4 nanoparticles exhibited the characteristic planes same as like CoFe2O4 and it indicated that the glycine surface modification did not affect the crystal structure of the particles (Figure. 2a.) The elemental compositions of the nanoparticles were estimated by using EDX detector and the results were shown in Figure. 2b. The CoFe2O4 nanoparticles showed 78.93 wt% of Fe, 11.88 wt% of Co, 7.96 wt% of O, and 1.23 wt% of nitrogen. The Gly/CoFe2O4 nanoparticles contained 58.15 wt% of Fe, 19.21 wt% of Co, 20.93 wt% of O and 1.70 wt% of nitrogen. The increase of nitrogen weight percentage indicated that the successful conjugation of glycine on CoFe2O4 nanoparticles. The magnetic sensitivity of the nanoparticles was measured using the VSM and the results were shown in Figure. 2c. From the curve the saturation magnetization (Ms) value of CoFe2O4 nanoparticles was found to be 50.232 emu/g and the Gly/CoFe2O4 nanoparticles showed the magnetization value was 49.24 emu/g.
Figure 2b: Elemental analysis of NPs by energy dispersive X-ray (EDX) of (a) CoFe2O4 and (b) Gly/CoFe2O4 nanoparticles.
The glycine group was estimated at 15.5 nmol glycine/mg of nanoparticles. In our study, the amine density on the nanoparticle surface was estimated to be 0.437 μmol/mg nanoparticles. This amine content corresponds to about 15.6 nmol glycine/mg of nanoparticles. To study the bacterial collection efficiency, bacterial isolates were serially diluted up to 5 × 106 CFU/mL and incubated with CoFe2O4 and Gly/CoFe2O4 nanoparticles. From the results, we found that the Gly/CoFe2O4 nanoparticles exhibit maximum 92% ± 1 of E. coli. The CoFe2O4 nanoparticles exhibited 25 ± 3.01% of E. coli and their binding were confirmed with TEM and plating method (Figure. 3). The glycine molecules in the nanoparticles surface offered amino terminus for the non-specific bacteria binding. The non-specifically collected bacteria from the water samples were lysed with lytic buffer and the specific E. coli surface marker was allowed to react with specific substrate such as 4-methylumbelliferyl β-D-glucuronide hydrate and the reaction chemistry was shown in Figure. 3a. Initially the experimental parameters such as substrate concentration, incubation time were optimized. To determine the concentration of β-gal released from the lysed bacteria cells, varying concentrations of bacteria (0 mL−1, 105 mL−1, 106 mL−1 and 107 CFU mL−1) were taken in eppendroff tube and mixed with 2 mL of lytic buffer and vigorously mixed for 5 min. After that, the lysate was mixed with 50μL of 4-methylumbelliferyl β-D-glucuronide hydrate (100 mm) and the absorbance was measured at different time intervals 0 min, 10 min, 20 min, 30 min and 60 min. Same kind of experiment also performed using nanoparticles, the enzyme activity was measured in different time intervals. We found the intensity was same after 20 min in both samples. So we fixed 20 min is enough for the assay.
Figure 3: The TEM images of the E. coli captured Gly/CoFe2O4 NPs and the plating method based confirmations.
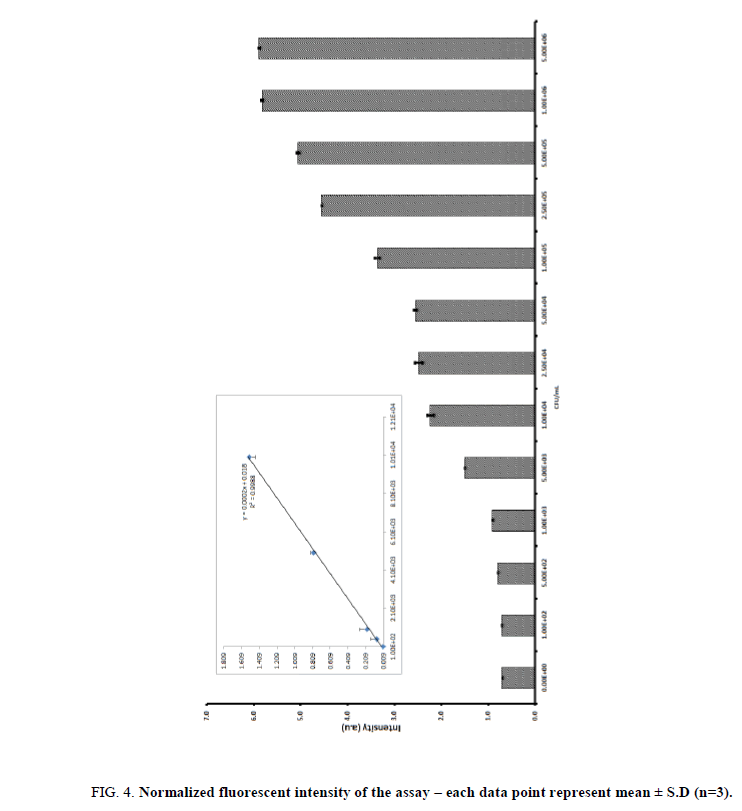
We also optimized the substrate concentration for this study we used increasing concentrations of E. coli (107 CFU/mL−1). And it was incubated with nanoparticles for 10 min, followed by lysis and magnetic separations. The lysate was incubated with various concentrations of 4-methylumbelliferyl β-D-glucuronide hydrate (25 mm to 150 mm in DMSO). The absorbance increased with increasing concentration of substrate and tended to level off after a concentration of 100 mM. Hence, a 4-methylumbelliferyl β-D-glucuronide hydrate concentration of 100 mM was selected for further enzymatic experiments. Under optimal conditions, the glycine coated nanoparticles were introduced to detect different concentration of E. coli. The glycine conjugated CoFe2O4 nano particles were exposed to different bacteria concentration ranging between 1 × 101~ 5 × 106 CFU/mL. The capture bacteria were washed with PBS buffer and followed by lysis with lytic buffer for 10 min and the lysate was collected using magnet and also mixed with 4-methylumbelliferyl β-D-glucuronide hydrate and the red colour product was transferred into a 96-well plate to obtain the fluorescent readout (excitation wave length 365 nm and the emission wave length 445 nm). The enzymatic product fluorescent intensity was increased with the increasing bacterial concentration and the result was shown in Figure. 4. Based on the absorbance, we could reproducibly detect bacteria concentration at 1 × 102 CFU·mL−1 (p<0.05). The linearity was obtained from 1 × 102 ~ 1 × 104 CFU/ml (R2=0.9944). The minimum detectable concentration was 1 × 102 CFU/mL and the inter-intra-assay cumulative values (CV) obtained was 5.03%, 6.23%, 6.50%, 5.34%, 5.11%, and 7.21% for a range between 1 × 101 CFU/mL ~ 1 × 106 CFU/mL. These results indicated that CV values were less than 10% hence the proposed method was highly reproducible. The specificity of the assay was evaluated using common pathogenic bacteria strains as competitors, including Salmonella enterica (S. enterica) and Staphylococcus aureus (S. aureus). The bacteria concentration of each strain in separate (106 CFU/mL) or mixed with E. coli solutions and the results indicated that the specific colour changes, absorbance were observed only with the presence of E. coli cells and it demonstrated this approach has a good specificity toward E. coli. The developed nanoparticles assay was then applied for the detection of bacteria in water sample. The water sample was spiked with 5 × 102, 5 × 103, 1 × 104, 1 × 105 and 5 × 105 CFU/mL of bacteria. The assay showed the average fluorescent intensity value (a.u) of bacteria without water spike such as 0.082 ± 0.02, 0.79 ± 0.04, 1.521 ± 0.06, 2.66 ± 0.51 and 4.350 ± 0.50. The bacteria spiked water samples showed the values were 0.080 ± 0.03, 0.80 ± 0.02, 1.454 ± 0.07, 2.60 ± 0.9, and 4.35 ± 0.5. Based on the results we found the assay is highly suitable for bacterial detections in water samples.
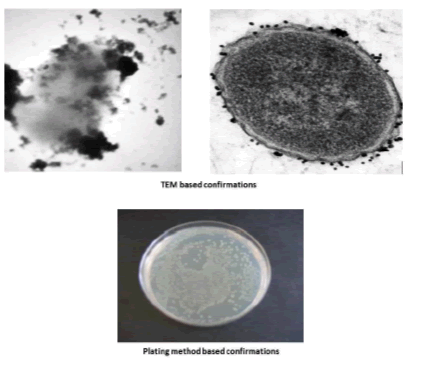
Figure 4: Normalized fluorescent intensity of the assay ? each data point represent mean ± S.D (n=3).
Conclusion
In conclusion, the glycine passivated cobalt ferrite nanoparticles were successfully synthesized and it was used as diagnostic tool for E. coli detections. The E. coli detection was carried out based on enzyme released from bacterial cell surface as a marker. The developed method is rapid, sensitive and avoids the pelleting and centrifugation steps and provided stable signal, flexible platform and short incubation time.
References
- Pandey PK, Kass PH, Soupir ML, et al. Contamination of water resources by pathogenic bacteria. AMB Express. 2014;4(1):51.
- Cabral JP. Water microbiology: Bacterial pathogens and water. Int J Environ Res Public Health. 2010;7(10):3657-703.
- Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Crit Rev Microbiol. 2002;28(4):371-409.
- Lleo MM, Bonato B, Tafi MC, et al. Molecular vs. culture methods for the detection of bacterial faecal indicators in groundwater for human use. Lett Appl Microbiol. 2005;40(4): 289-94.
- Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: Application to soil environment. Res Microbiol. 2000;151(3):167-77.
- Barghouthi SA. A universal method for the identification of bacteria based on general PCR primers. Indian J Microbiol. 2011; 51(4):430-44.
- Alharbi KK, Al-Sheikh YA. Role and implications of nanodiagnostics in the changing trends of clinical diagnosis. Saudi J Biol Sci. 2014;21(2):109-17.
- Jain KK. Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta. 2005;358(1):37-54.
- Aguilar-Arteaga K, Rodriguez JA, Barrado E. Magnetic solids in analytical chemistry: A review. Anal Chim Acta. 2010;674(2):157-65.
- de Dios AS, Diaz Garcia ME. Multifunctional nanoparticles: Analytical prospects. Anal Chim Acta. 2010; 666(1):1-22.
- Gao J, Gu H, Xu B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc Chem Res. 2009;42(8):1097-1107.
- Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2013;64:1532-55.
- Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 28;18(1):26-30.14.
- Parveen S, Misra R, Sahoo SK. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147-166.
- Kaiser E, Colescott RL, Bossinger CD, et al. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34(2):595-8.
- Viswanathan K, Raj GD, Vadivoo VS, et al. Development of antibiotic selection kit towards veterinary applications using glycine passivated magnetic particles. Biosens Bioelectron. 2014;51:47-54.