Research
, Volume: 15( 3) DOI: 10.37532/0974-7435.2019.15(3).191Effects of Differently Processed Cocoyam (Colocasia esculenta (L.) Schott) Inflorescence on Hematological and Histopathological Parameters of Albino Rats
- *Correspondence:
- Nweke Chijioke Joel , Department of Statistics, Alex-Ekwueme Federal University, Ndufu-Alike, Ebonyi State, Nigeria, E-Mail: cj_nweke@yahoo.com
Received: May 01, 2019; Accepted: May 21, 2019; Published: May 28, 2019
Citation: Okechukwu KE, Jane A, Joel NC. Effects of Differently Processed Cocoyam (Colocasia esculenta (L.) Schott) Inflorescence on Hematological and Histopathological Parameters of Albino Rats. Biotechnol Ind J. 2019;15(3):191.
Abstract
Effects of differently processed cocoyam inflorescence on hematological and histopathological parameters of albino rats were investigated. Cocoyam inflorescence was processed by blanching (water), soaking at 100°C (20 min), boiling (10 min) and drying (sun drying). Unprocessed (fresh) samples served as the control. The experiment included six groups of animals. Rats fed graded doses (100 mg/kg) of fresh, boiled, soaked and unblanched cocoyam inflorescence had significant (p<0.05) reduction in packed cell volume (PCV), red blood cell (RBC) and white blood cell (WBC) count. Rats fed boiled and blanched samples had significant (p<0.05) reduction in hemoglobin content by 14.4% and 29% while rats fed soaked, unblanched and fresh samples showed significant (p<0.05) increase in hemoglobin content from 9.67 in normal controlled rat to 10.07, 10.32 and 16.9 1g/dl respectively at 100 mg/kg dose. The relative liver and kidney weights of rats fed fresh, soaked, blanched and unblanched cocoyam inflorescence were significantly (p<0.05) higher than the relative liver and kidney weights of normal controlled rat while rat fed boiled sample showed no significant (p>0.05) difference in relative liver and kidney weights of the normal controlled rats. Light microscopic examination of liver and kidneys in controlled rats showed normal histological structure when compared to those of rats fed processed samples. The study has clearly demonstrated that boiling (10 min) and sun drying of cocoyam inflorescence are capable of improving hematological and histopathological parameters of rat.
Keywords
Cocoyam inflorescence; Hematological parameters; Processing
Introduction
Green leafy vegetables contain a bioactive compound (phytochemicals) that have potentials in helping to reduce the risk of several deadly diseases in man. High consumption of green leafy vegetables, therefore, plays a vital role in human nutrition and health [1]. Such bioactive and health-promoting compounds include alkaloids, flavonoids, saponin and tannins and are associated with the reduction in the risk of cancer and other degenerative diseases [2]. Some anti-nutritional phytochemicals such as tannin and phytate exhibit protective effect, thus making them serve a dual purpose of reducing some essential nutrients and protecting the body against a number of biochemical, physiological metabolic disorders [3]. These protective effects of vegetables include their antioxidant activities. Antioxidants help the body to develop the capacity to fight diseases by boosting the body’s immunity. This role of antioxidant coupled with health consciousness among other factors appears to have shifted research attention to the identification of biologically active components in foods that exhibit potentials to reduce the risk of diseases. Various traditional post-harvest processing treatments such as soaking in hot water, boiling, squeeze washing with water, and hot water blanching may, however, lead to losses of some of the characteristics which initially made green leafy vegetable consumers delight [4].
Development of normocytic hypochromic anemia as a result of significant reduction in hemoglobin (Hb) concentration, Packed Cell Volume (PCV) and Red Blood Cell (RBC) counts has been reported [5,6]. Food has traditionally been viewed as a means of providing nutrients for normal growth and development [7]. However, presently, science has obviously identified an additional role of some plant foods in reducing disease risk. In the process of widespread consumption of vegetables, individuals have learned that green leafy vegetable foods have a greater impact on health than previously known.
Cocoyam inflorescence is one of such vegetable food with healthful and nutritional benefit. Cocoyam inflorescence is the flowering part of Cocoyam plant. Cocoyam inflorescence occurs on a stout peduncle, shorter than leaf stalks, with pale yellow color and about 20 cm long. Cocoyam inflorescence is an edible flower. It is consumed as delicacies in the southern part of Nigeria. Cocoyam inflorescence is locally referred to as “Akpuru ede”, ?Opere”, “Efuru ede”, ?Ogbala ede”, ‘Opi ede’, ‘Orim ede’ and‘Umi ede’ by the Igbo tribes. Among the four species of cocoyam that produce inflorescence, only NCE005 is used for this work and is the most common species used in the southern parts of Nigeria and has the highest yield during the season. Generally, processing improves the bioavailability of plant proteins include vegetable proteins.
Traditionally fresh cocoyam inflorescence is used fresh as a culinary vegetable. It is also dried, milled and used as a spice in some communities to impact peculiar flavor. However, the use of fresh cocoyam inflorescence is usually associated with irritating sensation in the mouth and throat due to the presence of oxalate. Elimination of oxalate and other toxic substances in cocoyam inflorescence through processing will enable gainful exploitation of other useful inherent components. Many food processing techniques have been highlighted as a possible means of reducing or totally eliminating the antinutrients in plant foods. However, there is a paucity of information on hematological and histopathological parameters of rat fed differently processed cocoyam inflorescence. The purpose of this study, therefore, is to determine the effect of differently processed cocoyam inflorescence on the hematological and histopathological parameters of rats (FIG. 1).
Materials and Methods
Sources of materials
Fresh samples of Cocoyam (Colocasia esculenta (L.) shoot inflorescence NCe005 used for this study were procured from cocoyam section of Natural Root Crop Research Institute (NRCR), Umudike, Abia State where it was also identified.
Sample preparation
Fresh cocoyam inflorescence was harvested, washed with potable water, drained and allowed the water on the surface to dry under the fan for 30 to 40 min at room temperature. The washed air dried cocoyam inflorescence were sliced and divided into five portions of 2 kg each. Each portion was subjected to different processing treatment described as follows;
The first portion (2 kg fresh sample) served as control and was not given any treatment. This portion was blended using a Kenwood blender-BL300/ISL350 series to obtain the wet milled fresh sample. It was packaged in a plastic container and stored in the freezer until used for analysis.
The second portion was blanched in hot water (98% for 4 min). The time for adequate blanching of the inflorescence was determined as described by Luh and O’Neal [8]. This blanched sample was sun dried for a period of four days. The dried sample was milled using Kenwood blender-BL300/BL350series. They were packaged in a plastic container and stored in a refrigerator (10°C) until used for analysis.
The third portion (2 kg) was sun-dried (unblanched dried) for a period of four days. The dried sample was milled, packaged and stored as previously described until used for analysis.
The fourth portion was soaked in hot water (100°C) for 20 min. The sample was allowed to air dry after which, it was sun dried for a period of four days. The sample was milled and stored as described above until used for analysis.
The fifth portion was boiled at 100°C for 10 min and allowed to cool and air dried. The boiled samples were sun dried for a period of five days. The dried samples were milled and stored as previously described above until used for analysis.
Animal procurement and care
The animals were housed in well-ventilated cages containing wood shavings for bedding. All the animals were fed standard pelletized grower’s mash (UAC Vital Feed, Jos, Nigeria) and portable water ad libitum. They were maintained under normal environmental temperature (26 ± 2°C) with normal 12:12 hour dark/light cycle. The animals were acclimatized for 7 days. The weight of each animal was taken prior to the commencement of administration of the test samples. The environment was cleaned and disinfected regularly. Soiled wood shavings were replaced within 5 days interval. The feed and water containers were washed regularly. Each rat was marked for identification. The experiment was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the US and guidelines (NIH Publication No. 85-23, reversed in 1985).
Experimental design
Albino Rats (Wistar strain) of both sexes between 3-4 months old weighing between 100 g to 245 g were used. Sixty rats were divided into six groups. The first group was given the normal rat chow only which served as the control while Groups 2-6 were given treated samples (fresh, boiled, soaked, water blanched and unblanched dried samples). The samples were administered to the animals once daily while their normal feeding continues. Rats in groups 2-5 were administered with graded doses of the inflorescence powders dispersed in 10 ml of water while group 6 was fed with wet milled fresh sample according to their weight. Feeding was done by intubation method at the doses of 10, 100, 1600, 2900 and 5000 mg/kg body weight. All the groups of animals were fed the normal rat chow while the samples were being administered to them. The samples were fed to the rats for a period of 10 days as described by Lork [9]. The body weights of the animals were taken at the 5th and 10th day. At the end of the experiment, blood samples of the animals were collected for hematological analysis. The blood of the animals was collected by aucular puncture [10]. Following the blood collection, the animals were euthanized and exsanguinated under chloroform anesthesia and their liver and kidney collected for relative organ weight (ROW) and histopathological examination.
Analysis
Hematological analysis: A portion of the blood samples collected from the rats was dispensed into Ethylene Diamine-Tetra-Acetic Acid (EDTA) anticoagulant bottle from where Red Blood Cell (Erythrocyte) count, White Blood cell (Leucocyte) count, Differential Leucocyte count, Relative Volume of corpuscles to plasma (Packed Cell Volume or Haematocrit) and hemoglobin count were determined.
Histopathological examination: The tissue samples viz kidneys and liver from one animal in each group were collected in 10% Neutral buffered formalin (NBF) and processed by routine paraffin embedding method. The tissue sections of 3 to 5 microns size were stained by routine Haematoxylin and Eosin staining as suggested by Chauhan et al. [11].
Data analysis
Experimental design: The experiment was laid out in Completely Randomized Design (CRD). One way analysis of variance (ANOVA) was used to analyze the data. Means separation was by Duncan multiple range test as described by Klaus and Oscar [12]. Results were expressed as Mean ± SD (standard deviation) of triplicate determination. The data analysis was aided by SPSS version 20.
Results and Discussion
The Red Blood Cell (RBC), Packed Cell Volume (PCV), hemoglobin (HB), White Blood Cell (WBC) and Differential Leucocytes Count (DLC) of the rats fed different doses of cocoyam inflorescence extract processed by different methods are shown in TABLE 1. Animals fed 100, 200 and 6400 mg/kg body weight doses of processed samples showed significant (p<0.05) decreases in PCV, RBC and HB levels respectively but the decrease was more in animals fed the blanched samples due probably to the presence of residual antinutrient components. The significant (p<0.05) decreases in RBC and HB were lower than the values shown by the animals fed the control (6.76-9.20) and reference standard (11.5-16.1) values [13]. The PCV of animals fed the processed samples were significantly (p<0.05) lower than that of animals fed control sample but within the range of values (37.6-50.6) recommended by Bru et al as the reference standard [13]. Akinuga et al. reported similar reductions in RBC, PCV, and HB in rats fed ethanol leaf extract of Gongronema latifolium [14]. Animals fed fresh cocoyam inflorescence had 3.96, 5.00 and 7.18 mm3 red blood cell count respectively. Nwankpa et al and Sodipo et al reported similar observation in RBC of rats fed fresh extract of Phyllanthus amarus leaf extracts and A. solanidine [15,16]. The significant (p<0.05) decrease in PCV, RBC and HB could probably be due to the effect of metabolites from the plant. At 6400 mg/kg dose, rats fed the boiled and fresh sample extracts showed significant (p<0.05) increase in RBC (6.32 and 7.18 mm3) respectively due probably to the accumulation of bioactive components as well as adjustments in the body system of the animals.
The PCV of rats fed fresh and unblanched dried sample extracts did not differ (p>0.05) from that of controlled animals at doses of 100 and 200 mg/kg body weight (TABLE 1). Nwankpa et al. and Sodipo et al. reported similar observation in rats fed Phyllanthus amarus and aqueous extract of Solanum macrocapus [15,16]. The rats fed fresh sample extract showed significantly (p<0.05) higher hemoglobin count than those fed the control sample at all the doses of administration. Animals fed unblanched sample extract had hemoglobin count that did not differ significantly (p>0.05) from the hemoglobin of animals fed the normal rat chow (control). Rats fed blanched sample extract recorded significantly (p<0.05) lower hemoglobin count at all doses of administration than rats fed other processed samples of extract. The blanching treatment may have exposed the animals to the toxic components of the sample which produced metabolites that could have been responsible for the significant (p<0.05) reduction in hemoglobin, packed cell volume and red blood cell count of the animals.
| Processed samples | PCV (%) | RBC (X103/MM3) | WBC (X103/MM3) | HB (gHb/100m/s) | Differential Leucocyte count (%) | Doss (mg/kg) | |
|---|---|---|---|---|---|---|---|
| Lymphocyte | Neutrophil | ||||||
| Boiled | 27.0a ± 0.71 | 5.626c ± 0.46 | 8.61b ± 3.24 | 8.28b ± 0.66 | 82.20b ± 8.32 | 19.20b ± 0.84 | 100 |
| Soaked | 43.0b ± 6.52 | 6.56c ± 0.66 | 7.29a ± 0.85 | 10.07c ± 0.31 | 80.40b ± 2.88 | 22.90c ± 0.74 | 100 |
| Blanched | 27.8a ± 0.84 | 2.84a ± 0.62 | 15.36d ± 5.07 | 6.87a ± 0.82 | 86.60c ± 5.89 | 17.60a ± 1.14 | 100 |
| Un-blanched | 52.8d ± 1.10 | 3.51b ± 0.70 | 8.55b ± 0.48 | 10.32c ± 0.66 | 85.80c ± 5.26 | 20.50b ± 2.78 | 100 |
| Fresh Sample | 48.0c ± 1.58 | 3.96b ± 1.23 | 8.48b ± 1.58 | 16.91d ± 2.07 | 62.80a ± 3.56 | 35.60d ± 1.51 | 100 |
| Control | 55.0d ± 2.55 | 6.49c ± 1.08 | 10.6c ± 1.48 | 9.67c ± 0.43 | 78.00b ± 1.58 | 22.30c ± 2.49 | 100 |
| Boiled | 38.0b ± 1.00 | 5.19b ± 0.51 | 7.30a ± 1.20 | 9.92a ± 0.71 | 80.00b ± 5.48 | 28.00c ± 3.69 | 200 |
| Soaked | 44.8c ± 0.84 | 4.79b ± 0.62 | 7.73b ± 0.36 | 9.86a ± 1.03 | 80.40b ± 10.36 | 22.90b ± 3.68 | 200 |
| Blanched | 25.2a ± 1.30 | 3.22a ± 0.44 | 13.01e ± 1.38 | 9.00a ± 0.12 | 77.00b ± 2.92 | 20.40a ± 1.14 | 200 |
| Un-blanched | 57.0d ± 1.87 | 4.92b ± 0.69 | 8.39c ± 1.05 | 11.12b ± 0.87 | 87.20a ± 6.38 | 21.90a ± 2.75 | 200 |
| Fresh sample | 53.4d ± 1.14 | 5.00b ± 1.33 | 6.63a ± 0.57 | 17.31c ± 0.48 | 67.80a ± 1.92 | 33.60e ± 2,70 | 200 |
| Control | 53.6d ± 6.35 | 7.06c ± 1.56 | 12.3d ± 0.77 | 11.31b ± 1.20 | 72.40a ± 3.44 | 29.79d ± 3.56 | 200 |
| Boiled | 40.4d ± 1.14 | 6.32c ± 0.58 | 4.01a ± 0.71 | 8.28a ± 0.83 | 83.60b ± 8.91 | 21.50c ± 1.12 | 6400 |
| Soaked | 37.2c ± 0.84 | 5.46b ± 0.71 | 4.49a ± 0.86 | 9.40b ± 0.73 | 79.00b ± 7.45 | 20.60c ± 1.98 | 6400 |
| Blanched | 22.2a ± 1.30 | 4.63a ± 0.83 | 4.22a ± 0.55 | 7.66a ± 0.72 | 83.20b ± 4.15 | 18.60b ± 1.95 | 6400 |
| Un-blanched | 34.0b ± 1.23 | 4.64a ± 0.47 | 6.24b ± 0.43 | 8.84a ± 0.80 | 73.60a ± 4.04 | 16.80a ± 1.30 | 6400 |
| Fresh sample | 46.6e ± 1.14 | 7.18d ± 0.79 | 5.95b ± 0.90 | 15.64c ± 0.96 | 71.80a ± 1.64 | 25.60d ± 1.14 | 6400 |
| Control | 47.2e ± 1.92 | 7.49d ± 1.33 | 15.24c ± 1.46 | 8.60a ± 1.39 | 78.60b ± 2.41 | 22.80c ± 1.92 | 6400 |
Values are means of triplicate determinations ± standard deviation. Means in along the same column with different superscripts are significantly different (p<0.05)
Table 1: Hematological indices of rats fed different levels of differently processed cocoyam inflorescence.
At the middle dose of 200 mg/kg body weight, increases were observed in PCV, RBC and HB of animals relative to the values shown at 100 mg/kg dose of administration in all the treatment groups except in the HB count of rats fed the soaked sample extract. These increases could be attributed to the effect of higher doses of bioactive compounds in the sample extract which may have increased production of the bone marrow thus improving the haematopoetic activities of the cells and hence the erythrocyte [15,17]. This is in agreement with the observation of Okigbo and Ajalie, Kumar and Kuttan and Tilak et al. who reported that phytochemicals in vegetables or plants ameliorate the disintegration of deoxyribonucleic acid, leading to an increase in protein synthesis and cell proliferation [18-20]. This may suggest that the consumption of both fresh and processed cocoyam inflorescence may enhance blood cell production. Similar decreases had been reported by Ephraim et al., Berezi et al. and Ojo et al. in rats fed extract of O.gratissimum and CCl4 [21-23]. Rats fed fresh and unblanched dried samples showed higher significant (p<0.05) increase in PCV and HB values at 100 mg/kg doses relative to rats fed boiled, blanched and soaked sample extracts.
Rats which were fed fresh samples showed higher significant (p<0.05) increase in Hb values than rats fed the control samples. This could be due probably to primary metabolites such as total proteins, lipids, and carbohydrates, secondary metabolites like alkaloids, flavonoids, saponin and tannins, minerals especially the high level of it iron content and as well magnesium, vitamins which include Vitamin C, and Vitamin B2 which possesses antioxidant activities thus making them to serve a protective agents to the body against a number of biochemical, physiological metabolic disorders [3]. Bushra et al. reported similarly that Acacia modesta wall leaves contained total proteins, lipids, carbohydrates, total polyphenols, flavonoids, polysaccharides and glycosaponins which contributed to improving a variety of pharmacological activities like anti-diabetic and anti-cancer effects [24].
All rats fed the processed sample extracts except the blanched samples showed significantly (p<0.05) lower WBC than rats fed the control sample extract (TABLE 1). The significant decrease in WBC could be due to the presence of phytochemicals that exhibited antioxidant properties thus protecting the immune system. Akinnuga et al. and Ojo et al. reported that administration of aqueous extract of T. officinale significantly decreased the WBC [14,23]. This suggests that the consumption of fresh and processed cocoyam inflorescence may also serve to protect the human immune system by exhibiting antioxidant properties.
More so, the effect of the extract from both processed and fresh samples on PCV, Hb, and WBC of rats was observed to be dose-dependent. This dose dependence explains the optimal dose at which the bioactive compounds could be used to an advantage. At 100 and 200 mg/kg doses of both fresh and processed cocoyam inflorescence extract administration, there were significant (p<0.05) increases in PCV, RBC, HB and decrease in WBC values of rats. At 200 mg/kg dose all the rats showed maximum levels of hematological values while at 6400 mg/kg dose there were significant (p<0.05) decreases in these hematological parameters. This observation agrees with the report of Ojo et al. who noted that bioactive compounds like saponin, triterpenes, carotenes, flavonoids, phenol, and alkaloid caused anemia, increase in leucocytes and suppressed the hemopoietic system during a 2-week administration of Ocimum gratissimum at a dose of 400 mg/kg [23]. In this research, the medium dose (200 mg/kg) appeared to give the best hematological result. This could be the dose at which most bioactive compounds were satisfactorily utilized by the animal (rats) such that the levels of bioactive compounds were not harmful to the animals.
The dose-dependent effects of cocoyam inflorescence extract in the tested animals suggest a cumulative action of the active ingredients present in the inflorescence. The increase in PCV, Hb, and decrease in WBC in rats fed cocoyam inflorescence extract especially the unblanched dried and fresh samples suggests that cocoyam inflorescence may have prophylactic and therapeutic effect on the body system as other herbs and spices like common dandelion, Nuclear latifolia Smith, Tridax procumbens L and Vernonia amygdalina Del that have been shown to possess similar antioxidant and hematological properties as claimed by traditionalist [25-28]. There was a significant (p<0.05) increase in the lymphocyte content of rats administered processed sample extract than that of rats fed the control sample at all doses while rats fed fresh sample extract showed a significant decrease in lymphocyte count (TABLE 1). This observation is in agreement with that of Nwankpa et al. and Sodipo et al. who reported lymphocytes of rat fed solanum extract to be higher (55.50%) than that of those fed the control (41.0%) [15,16]. The neutrophils content of the rats fed processed sample extract at all doses showed significant decrease than that of the control (TABLE 1) while the rats fed fresh sample extract had significant (p<0.05) increase in neutrophil than the control. This significant increase in lymphocytes and decrease in the neutrophil count of rats administered processed cocoyam inflorescence extract could be due to possible stimulation of the immune defense system induced by the presence of toxic components [29]. Ugochukwu et al. and Sodipo et al. noted that the presence of toxic compound causes increases in leucocytes and consequently the rupturing of neutrophil [30,31]. Sanni et al., Kumar et al., and Obianime et al. reported similar observations [32-34]. The significant decrease in the lymphocytes level and increase in the level of the neutrophils of rats fed fresh sample extract could be attributed to the high level of an antioxidant component such as flavonoid, carotenoid, steroid, phenol, and other phytochemicals present. These substances reduced phagocytes and White Blood Cell count (WBC). When phagocytes are reduced the ability of the neutrophils to function is reduced and it rupture will be prevented thereby leading to an increase in the level of neutrophil. However, the observed levels of lymphocytes and neutrophils in this work are within the reference standard (50.2-84.5 and 4.4-49.2) levels thus suggesting that even fresh cocoyam inflorescence is safe [13].
Effect of differently processed cocoyam inflorescence on histopathological indices of rats.
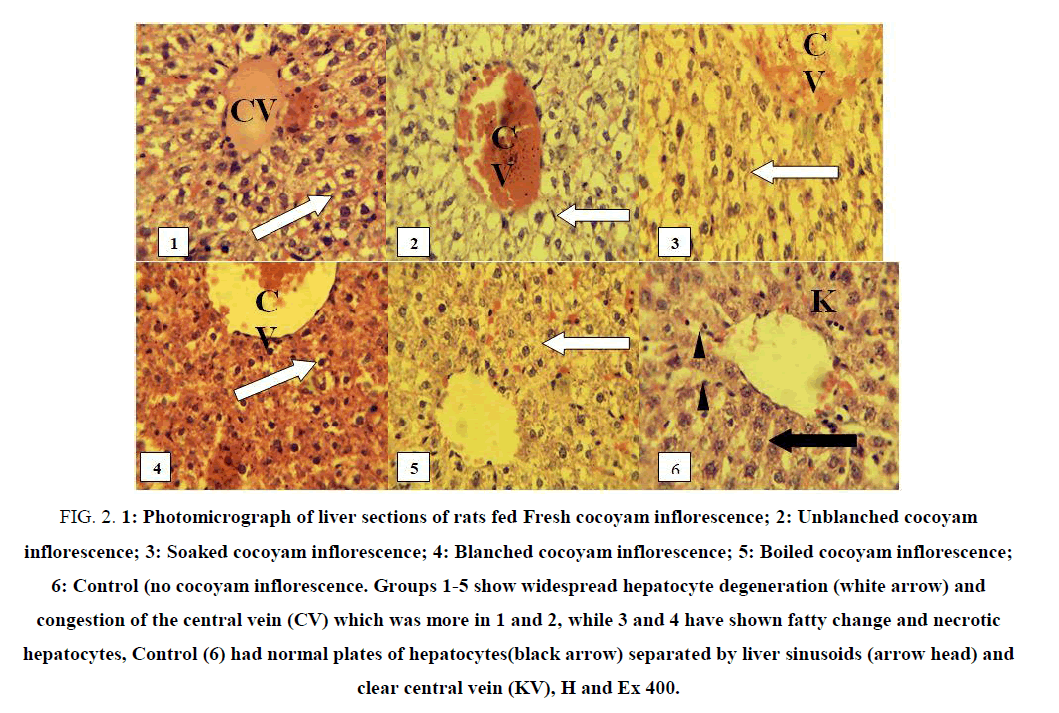
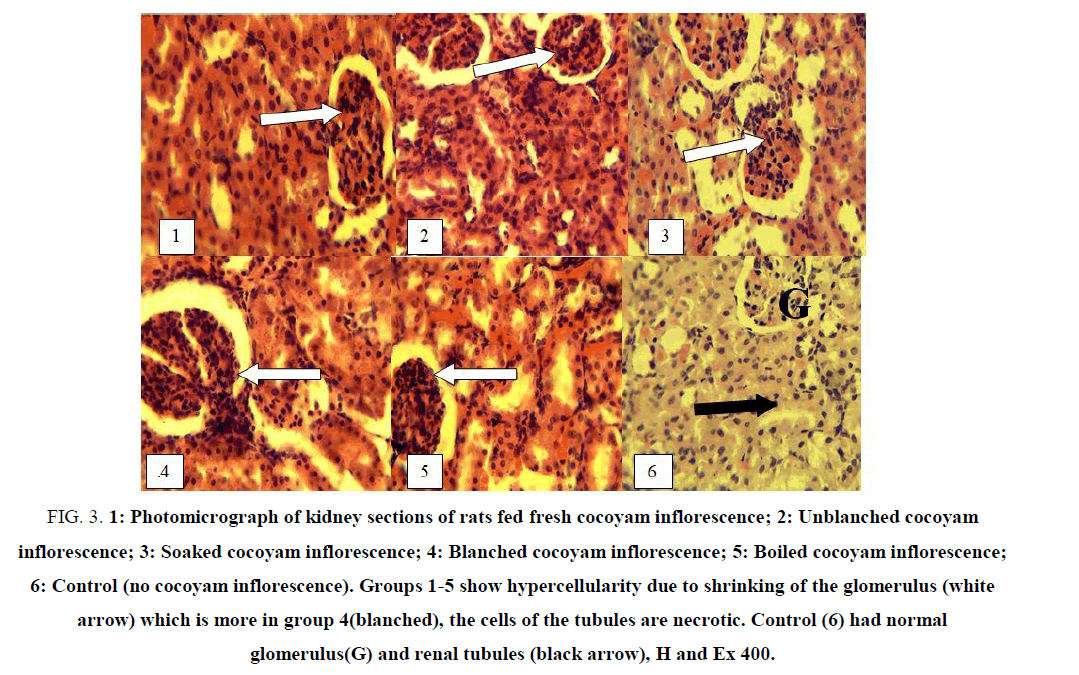
Light microscopic examination of the liver and kidneys of rats fed differently processed cocoyam inflorescence are shown in FIG. 2 and 3. Photomicrograph of liver and kidneys of control rats showed normal histological structures.
Figure 2: 1: Photomicrograph of liver sections of rats fed Fresh cocoyam inflorescence; 2: Unblanched cocoyam inflorescence; 3: Soaked cocoyam inflorescence; 4: Blanched cocoyam inflorescence; 5: Boiled cocoyam inflorescence; 6: Control (no cocoyam inflorescence. Groups 1-5 show widespread hepatocyte degeneration (white arrow) and congestion of the central vein (CV) which was more in 1 and 2, while 3 and 4 have shown fatty change and necrotic hepatocytes, Control (6) had normal plates of hepatocytes(black arrow) separated by liver sinusoids (arrow head) and clear central vein (KV), H and Ex 400.
Figure 3: 1: Photomicrograph of kidney sections of rats fed fresh cocoyam inflorescence; 2: Unblanched cocoyam inflorescence; 3: Soaked cocoyam inflorescence; 4: Blanched cocoyam inflorescence; 5: Boiled cocoyam inflorescence; 6: Control (no cocoyam inflorescence). Groups 1-5 show hypercellularity due to shrinking of the glomerulus (white arrow) which is more in group 4(blanched), the cells of the tubules are necrotic. Control (6) had normal glomerulus(G) and renal tubules (black arrow), H and Ex 400.
Liver photomicrograph
FIG. 2 (1) shows the photomicrograph of the liver of a rat fed fresh samples of cocoyam inflorescence. The liver showed centrilobular or areas of fatty change of the hepatocytes, with congestion of central vein. FIG. 2 (2) shows the photomicrograph of the liver of rat fed unblanched dried samples of cocoyam inflorescence. There was also congestion of the entire liver with more fatty change than what was observed in group one (rat fed fresh sample). These abnormalities or mild infection of the liver may be as a result of the toxic effect of the samples on the liver cells. However, the adverse effect on the liver tissue was not severe at the dosage and duration of administration as the liver cells were not dead but may recover fully if the toxic components are reduced or completely eliminated. Increase in the dose and duration of administration of these samples may eventually cause the death of the liver cells.
FIG. 2 (3) and 2 (4) show the photomicrograph of the liver of rats fed soaked and blanched samples of cocoyam inflorescence respectively. These showed severe liver damage as most of the hepatocytes surrounding the central veins are pyknotic, a sign of necrosis or cell death. Centrilobular (periacinar) degeneration or necrosis is common because this portion of the lobule received the least oxygenated blood and is very susceptible to hypoxia (reduced oxygen supply). This lobule or infected portion of the liver had the greatest enzyme activity that is capable of activating the chemical components of the samples into toxic forms. Therefore, the necrotic hepatocytes may be as a result of an increase in the toxic components of the samples which caused the inadequate supply of blood to the liver or the toxic effect of the metabolites produced by the enzymes present mainly in the centrilobular area of the liver.
The increased toxicological effect produced by the soaked FIG. 2 (3) and blanched FIG. 2 (4) sample extracts may be because the toxic components of the cocoyam inflorescence were exposed in a greater level by these two processing methods thereby causing the injury on the hepatocytes. Also, the rats fed blanched and soaked sample extracts recorded the least weight in the experiment, the highest pathological effect, and mortality. The liver is the site of metabolism in the body and its injury will definitely affect the production of proteins (albumin, globulin), glycogen storage and many other functions of the liver including detoxification. That may explain the reduction in weight and mortality recorded in these two groups.
FIG. 2 (5) shows the photomicrograph of the liver of rats fed boiled sample extract of cocoyam inflorescence. There were mild hepatocytes degeneration and no congestion of central vein. It was close to normal. The boiling method caused a significant decrease in the toxic components of the sample and release of plant nutrients mostly antioxidant components which had a restorative effect on the hepatocytes. This is similar to the observation of Nale et al. who reported a restorative change in the hepatocytes when extract of Carica papaya L seed was administered to rats [35]. Rats fed boiled samples also recorded the highest weight.
FIG. 2 (6) shows photomicrograph of the liver of rats fed normal rat chow (control). There were normal hepatocytes separated by a liver sinusoid and a clear central vein. The liver shows no sign of infection or abnormalities.
Kidneys
FIG. 3 shows the microscopic examination of the kidney of rats fed differently processed cocoyam inflorescence. The kidney of rats in all the groups (1-5) showed a sign of infections of the kidneys. There was shrinkage of glomerulus which gave the impression of increased cellularity. The nephrons (the tubules) are lined by necrotic cells indicative of toxic nephrosis. The lesion suggests toxic damage to the kidneys. Similar changes were reported by Bibu and Joy, Dehghani et al., and Nale et al., when gentamicin and taurine were administered to rats [35-37]. These effects occurred in all the kidneys especially rats that were fed blanched samples but the effect was mild in the kidney of rats fed boiled sample 5. The mild effect observed on the hepatocytes and tubular epithelial cells of rats in group 5 indicate that boiling method reduced the toxic components of the cocoyam inflorescence. The kidney section of rats in the control 6 group had normal glomerulus and structures of the uninfected kidney.
TABLE 2 shows the mean values of the absolute and relative liver and kidneys weights of rats fed differently processed cocoyam inflorescence. The results show that the relative weights of liver and kidney of rats fed processed samples extract were higher than that of rats fed the normal (control group). A similar result had been reported by Akah et al. and Saba et al. [6,38]. Rats fed blanched samples extracts showed significantly (p<0.05) higher relative weights of liver and kidney while rats fed boiled sample extracts showed liver and kidney weights that did not differ significantly (p>0.05) from that of the control group. The significant (p<0.05) increase in relative liver and kidney weights of rats fed processed sample extract is an indication of infection of the organs due probably to the presence of toxic components in those samples. The infection was severe in the organs of rats fed blanched sample extract followed by the organs of rats fed soaked samples but was mild in organs of rats fed boiled sample extract which did not differ (p>0.05) from that of the control group organs. The mild infection in the organs of rats fed boiled extract is reversible probably at the elimination of the toxic components by increasing the boiling time or antioxidant components of the sample or complete withdrawal of the sample extract containing the toxic substances since the cells are not yet necrotic. Therefore, blanching and soaking methods should not be used to process cocoyam inflorescence because samples processed by these methods contain higher levels of residual toxic components capable of eliciting damages in the body system. The boiling method having shown the least toxic effects with an increased weight gain of the rats is, therefore, the best effective method of processing cocoyam inflorescence for human consumption especially boiling for 10 to 20 min as the results of this research suggest.
| Processed Cocoyam inflorescence | Weight after 10 days | Absolute liver weight (g) | Relative liver weight (g/100g) | Absolut kidney weight (g) | Relative kidney weight (g/100g) |
|---|---|---|---|---|---|
| Boiled Sample | 184.48 | 6.22 ± 0.02 | 3.37a ± 0.06 | 1.32 ± 0.01 | 0.72b ± 0.01 |
| Soaked sample | 133.03 | 6.45 ± 0.15 | 4.84d ± 0.02 | 1.45 ± 0.08 | 1.08d ± 0.02 |
| Blanched sample | 138.05 | 6.75 ± 0.30 | 4.89d ± 0.00 | 1.56 ± 0.03 | 1.13e ± 0.02 |
| Unblanched sample | 161.99 | 6.30 ± 0.24 | 3.88b ± 0.01 | 1.40 ± 0.04 | 0.86c ± 0.01 |
| Fresh sample | 157.78 | 6.40 ± 0.31 | 4.05c ± 0.06 | 1.55 ± 0.06 | 0.98c ± 0.06 |
| Control | 189.20 | 6.20 ± 0.28 | 3.20a ± 0.07 | 1.31 ± 0.02 | 0.69a ± 0.01 |
Values are means of triplicate determinations ± standard deviation. Means in along the same column with different superscripts are significantly different (p<0.05)
Table 2: Effect of differently processed cocoyam inflorescence on relative organ (kidney and liver) weight.
Conclusion
The results of the study showed that processing treatment given to cocoyam inflorescence led to varying losses of hematological parameters and as well as histological degenerations. The losses notwithstanding could maintain hematological level that could not pose health treat. The degeneration of hepatocytes in liver and shrinkage of the glomerulus in the kidney of rats administered processed samples are reversible. The high level of hematological parameters in rats administered fresh cocoyam inflorescence indicated that fresh cocoyam inflorescence contained the appreciable level of nutrients and antioxidant potentials suggesting that processed cocoyam inflorescence could be useful in the preparation of food spices and management of chronic diseases.
References
- Odukoya OA, Inya-Agha SI, Segun FI, et al. Antioxidant activity of selected Nigerian green leafy vegetables. Am J Food Technol. 2007;2(3):169-75.
- Okwu DE. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci. 2005;1(4):375-81.
- Inyang UE, Ani JC. Effect of traditional processing methods on the nutrients and phytochemical contents in Lasianthera africana leaf residue. Int J Curr Res Bio Sci Plant Biol. 2015a;2(5):101-07.
- Adeboye AS, Babajide JM. Effect of processing methods on antinutrients in selected leaf. Nig Food J. 2007;25(2):77-87.
- Azeez IO, Oyagbemi AA, Oyeyemi MO, et al. Ameliorative effect of Cnidoscolus aconitifilius on alloxan toxicity in Wistar rats. Afr Health Sci. 2010;10(3):276-82.
- Saba AB, Oyagbemi AA, Azeez IO. Anti-diabetic and hematinic effects Parquetina nigrescens on alloxan induced type-1- diabetes and normocytic normochromia in Wistar rats. Afr Health Sci. 2010;10(3):276-82.
- Inyang UE, Ani JC. Ameliorative effects of Lasianthera africana leaf powder on hematological and biochemical parameters in alloxan-induced diabetic rats. Int J Curr Res Biosci Plant Biol. 2015b;2(6):43-9.
- Luh BS, Woodroof JG. Commercial vegetable processing. 1975.
- Erpenbach H, Gehrmann K, Lork W, et al. Process for making carboxylic acid halides. United States patent US 4,414,160. 1983.
- Wilson NH, Hardisty JF, Hayes JR. Short stem, subchronic and chronic toxicity studies. In: Hayes, A. W. (ed). Principles and methods of toxicology. Taylor and Frances, Philadelphia. 2001:917-56.
- Chauhan HVS. Vetrinary clinical and laboratory diagnosis. 3rd Edition Jaypee Publishers. New Delhi.
- Klaus H, Oscar K. Design and analysis of experiment. A John Wiley and Sons INC publication, USA. 2005:215-30.
- Mitruka BM, Rawnsley HM. Clinical biochemical and hematological reference values in normal experimental animals. 1977:256-60.
- Akinuga AM, Bamidele O, Ekechi P, et al. Effect of an ethanol leaf extract of Gongronema latifolium on hematological of some parameters in rats. Afr J Biomed Res. 2011;14:153-56.
- Nwankpa P, Agomuo EN, Uloneme GC, et al. Effect of Phyllantus amarus leaf extract on alterations of hematological parameters in Salmonellae typhi infested Wistar albino rats. Acad J Sci Res Essays. 2014;9(1):7-12.
- Sodipo OA, Adbulrahman FI, Sandabe UK, et al. Comparative hematological parameters of aqueous fruits extracts of Sanum macrocarpum, a-solanidine and standard lipid-lowering agents on trition-induced hyperlipidaemic rats. Wudpecker J Pharm Pharmacol. 2013;2(1):6-14.
- Maaz F, Javed S, Abdinn MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus, thonn on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol. 2007;113:503-09.
- Okigbo RW, Ajalie AN. Inhibition of some human pathogens with tropical plant extracts Chromolaeng odorata and Citrus aurantifolia, and some antibiotics. Int J Mole Med Adv Sci. 2005;1(1):41-8.
- Kumar KBH, Kuttan R. Chemopreventive activity of an extract of Phyllantus amarus against cyclosphosphamide-induced toxicity in mice. Phytomedical. 2005;7:494-500.
- Tilak KS, Veeranaiah K, Reju MP. Effect of ammonia, nitrite and nitrite on hemoglobin content oxygen consumption of fresh water fish, Cyprinus carpol (Linnaeus). J Environ Biotechnol. 2007;28(1):45-7.
- Ephraim KD, Salami HA, Osewa TS. Effect of Aqueous leaf extract of Ocimium gratissimum on hematological and biochemical parameters in rabbits. Afr J Biomed Res. 2000;3:175-79.
- Berezi EP, Monago C, Adelagun ROA. Hematological profile of rats treated with aqueous extracts of common dandelion leaf (Taraxacum officinale Webar) against carbon tetrachloride (CCl4) toxicity. Int J Biochem Biotechnol. 2013;2(1):263-67.
- Ojo OA, Oloyede OI, Ajiboye BO, et al. Effects of aqueous extract of Ocimium gratissimum on some hematological parameters of albino rats. Am Chem Sci J. 2014;4(1):74-81.
- Bushra S, Muhammad I, Hamid S, et al. Investigations of Acacia modesta wall leaves for in vitro anti-diabetic, proliferative and cytotoxic effects. Braz J Pharma Sci. 2018;54(2):2175-9790.
- Akpanabiatu MI, Umoh IB, Eyong EY, et al. Influence of Nuclear intifolia leaf extract on some Hepatic enzymes of rats fed on coconut oil and non-coconut oil meals. Pharm. Biol. 2005;43(2):153-57.
- Nwajo HU. Efficacy of aqueous leaf extract of Vernonia amydalina on plasma lipoprotein and oxidative stress in diabetes rat models. Nig J Physol Sci. 20(1-2):39-42.
- Nwajo HU, Oze G, Pkafor MC, et al. Hypolipidemic and bradycardia potentials of aqueous leaf extract Tridax procumben in Hypercholesteroollaemic-induced Albina rats. Int J Biotechnol Allied Sci. 2007;2(1):76-86.
- Arhoghro EM, Ekpo KE, Amosike EO, et al. Effect of aqueous extracts of bitter leaf (Vernonia amygdaline) on carbon tetrachloride (CCl4) induced liver damage in Albino Wistar Rats. Eur J Sci Res. 26(1):115-23.
- Evans WC. Trease and evans Pharmacognosy. 15th Ed. Harcourt publishers LTD. China. 2002:289-393.
- Ugochukwu NH, Babady NE. Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci. 2003;73(15):1925-38.
- Sodipo OA, Abdulrahman FI, Sandabe UK. The leukocytic response in exogenously induced hypercholestero laemic rats treats with aqueous fruit extract of Solauum macrocaipum Linn. J Life Environ Sci. 2010;11(2):666-75.
- Sanni FS, Ibrahim S, Esievo KAN, et al. Effect of oral administration of aqueous extracts of Khaya senegalensis stem bark on phenylhydrazine-induced anaemia in rats. Pak J Biotechnol Sci. 2005;8:256-58.
- Kumar R, Nataraju S, Jayaprehash C, et al. Effect of heat treatment on the stability of oxalic acid in selected plant foods. Ind J Natural Dietetics. 2006;43:337-40.
- Obianime AW, Aprioku JS, Esomonu C. The effect of aqueous Ocimium gratissimum leaf extract on some biochemical and hematological parameters in male mice. Asian J Biotechnol Sci. 2011;4:44-52.
- Nale LP, More PR, More BK, et al. Protective effect of Carica papaya L. seed extract in Gentamicin-induced hepatotoxicity and nephrotoxicity in rats. Int J Pharm Bio Sci. 2012;3(3):508-15.
- Bibu KJ, Joy AD. Therapeutic effect of ethanolic extract of Hygrophila spinosa T. anders on gentamicin-induced nephrotoxicity in Wistar rats. Ind J Experimental Biol. 2010;88:911-17.
- Dehghani, F, Namavar MR, Noorafshan A, et al. Evaluation of the kidney extract on gentamicin induced-nephrotoxicity in the rat. Kidney Res J. 2011;1:24-32.
- Akah PA, Alemji JA, Salawu OA, et al. Effect of Vernonia amygdalina on biochemical and hematological parameters in diabetic rats. Asian J Med Sci. 2009;1(3):108-13.