Original Article
, Volume: 12( 3)Effect of Asian Black Scorpion Heterometrus fastigiousus Couzijn Envenomation on Certain Enzymatic and Hematological Parameters
- *Correspondence:
- Mukesh Kumar C, Department of Zoology, Mahatma Gandhi Post Graduate College, Gorakhpur-273009, Uttar Pradesh, India, Tel: 073276 61110; E-mail: zoologyvr@rediffmail.com.
Received: August 16, 2017; Accepted: August 30, 2017; Published: September 04, 2017
Citation: Mukesh Kumar C. Effect of Asian Black Scorpion Heterometrus fastigiousus Couzijn Envenomation on Certain Enzymatic and Hematological Parameters. Res Rev Biosci. 2017;12(3):125
Abstract
In the present study, effect of Heterometrus fastigiousus Couzijn (Family: Scorpionidae) venom on alkaline phosphatase (ALP), acid phosphatase (ACP), lactic dehydrogenase (LDH) and glutamate-pyruvate transaminase (GPT) enzyme activity; and red blood cells (RBCs) count, white blood cells (WBCs) count, blood hemoglobin, mean corpuscular hemoglobin (MCH), packed cell volume (PCV) and plasma hemoglobin in experimentally envenomed albino mice was studied. Venom was obtained by electrical stimulation and its toxicity was determined in albino mice by subcutaneous envenomation. The LD50 was 18.6 mg kg-1 body weight mice. H. fastigiousus venom caused significant increase in ALP, ACP, LDH and GPT activity in liver tissue of albino mice. This venom reduced RBC count and increased WBC count, blood hemoglobin, MCH, PCV and plasma hemoglobin. Findings of this study will help to understand the mechanism of Asian black scorpion, H. fastigiousus venom toxicity.
Keywords
Heterometrus fastigiousus; Scorpion venom; Hemolysis; Envenomation
Introduction
Accidental scorpion sting is a serious health issue of poor communities in tropical and subtropical areas throughout the world. Out of 1500 scorpion species distributed throughout the world, only 50 scorpion species have been proved lethal to human [1]. The symptoms of scorpion envenomation depend on species, age, venom composition and the victim’s physiological response. Scorpion sting may induce local skin reactions, neurological, respiratory and cardiovascular disorders. Scorpion venom is a cocktail of various polypeptides with diverse pharmacological and physiological activities and exerts its effects by targeting ion channels [2]. Asian black scorpions belonging to genus Heterometrus (Family: Scorpiopnidae) are the largest scorpions living in Southeast Asian regions and are responsible for most of the accidental stings after their equivalents of family Buthidae. The toxic effect of these scorpion venoms and mechanism by which envenomation exerts its effects has not been clearly known. However, several scientific groups have reported pharmacological effects of some asian black scorpions. H. scaber venom causes prolong and sharp burning sensation around the site of sting [3]. Palmaneus gravimanus envenomation results in localized irritation, edema and itching [4]. H. fulvipes venom induces hemotoxic effects and inhibits acetylcholineesterase activity [5]. H. bengalensis venom produces irreversible nerve blockage [6]. H. longimanus and H. spinifer venoms produce contractile responses in rat anococcygeus muscle [7]. Black scorpion venoms contain high concentration of acetylcholine and nor-adrenaline, and cause reversible contraction of chick biventer cervicis muscle by cholineregic and adrenergic action [8]. P. gravimanus envenomation increases glucose, creatinine, blood urea nitrogen, alanine aminotransferase, creatine phosphokinase and lactic dehydrogenase and decreases total protein, uric acid, cholesterol, calcium and phosphate in serum of albino mice [9]. In the present investigation, the effect of black scorpion H. fastigiousus venom was studied for its effects on enzymatic and hematological parameters in albino mice after experimental envenomation.
Experimental Procedures
Isolation of H. fastigiousus venom
Living scorpions H. fastigiousus were purchased from Eastern Scientific Emporium, Gorakhpur, UP, India. Venom was obtained by electric stimulation of telson, dissolved in phosphate buffer (50 mM, pH 7.2) and centrifuged (MP01, Tarson Co., India) at 3,000 × g and 4°C for five minutes. The supernatant was collected, lyophilized and stored at 4°C until use. The venom protein content was estimated by Lowry et al. method [10].
Toxicity determination
H. fastigiousus venom was injected in mice weighing 25 g ± 5 g subcutaneously and LD50 was determined by Kankonkar etal. method [11]. Median lethal dose (LD50) was determined by injecting 0.1 ml of different dilutions of venom proteins subcutaneously. For each dose, four albino mice were used and mortality in experimental animals was recorded after 24 h of treatment. The LD50 represented dose at which half of the tested animals were died.
Experimental protocol for hematological and enzymatic assays
Three sets of albino mice weighing 25 g ± 5 g were used to study the effect of scorpion venom. Animals of the first set consisting of 12 albino mice were injected with 40% of 24 h LD50 and those of second set also consisting of 12 albino mice were injected with 80% of 24 h LD50 of scorpion venom subcutaneously. Mice of both sets were sacrificed 8 h after envenomation for hematological and enzymatic analysis. The third set consisted of six mice receiving only phosphate buffer (50 mM, pH 7.2) were used as control. At the end of experimental period, mice were anesthetized using vapours of ether. Blood was collected by cardiac puncture in tube containing anticoagulant ehylenediaminetetraacetic acid (EDTA) and used for hematological analysis. The liver was taken out by dissecting the animal for enzymatic analysis.
Hematological analysis: Determination of RBC count, blood hemoglobin, MCH, WBC count, PCV and plasma hemoglobin was done according to Dacie and Lewis method [12].
Enzymatic analysis: Determination of alkaline phosphatase (ALP) and acid phosphatase (ACP) activity: ALP and ACP enzyme activity in liver tissue was determined by Bergmayer’s (1967) method. A 50 mg of liver tissue was homogenized in 1 ml 0.9% sodium chloride solution and centrifuged at 5,000 g for 15 min at 0°C. The supernatant was used as enzyme source. For ALP enzyme activity determination, in 0.1 ml enzyme source, 1 ml alkaline buffer substrate was added, mixed and incubated for 30 min at 37°C. A 5 ml aliquot of 0.02 M NaOH was then added to the incubation mixture. For ACP enzyme activity determination, in 0.2 ml enzyme source, 1 ml acid buffer substrate was added, mixed and incubated for 30 min at 37°C. Now, 4 ml NaOH (0.1 M) was added to the incubation mixtures. The intensity of yellow colour developed was measured at 420 nm. A standard curve was drawn with different known concentration of p-nitro phenol. Enzyme activity was expressed as µ mol p-nitrophenol formed 30 min-1 mg-1 protein.
Determination of lactic dehydrogenase (LDH) activity
LDH activity in liver tissue was determined by Annon’s [14] method. A 50 mg of tissue was homogenized in 1 ml phosphate buffer (0.1 M, pH 7.5) in ice bath and centrifuged at 10,000 g for 30 min at 4°C. The supernatant was used as enzyme source. In 0.05 ml enzyme source, 0.5 ml pyruvate substrate was added and incubated at 37°C for 45 min. Now 0.5 ml of 2, 4-dinitrophenylhydrazine solution was added to mixture. After 20 min, 5 ml NaOH (0.4 M) was added and left for 30 min at room temperature. The absorbance of reaction mixture was measured at 540 nm. Enzyme activity was expressed as µ moles of pyruvate reduced 45 min-1 mg-1 protein.
Determination of glutamate-pyruvate transaminase (GTP) activity
GPT activity in liver tissue was determined by Reitman and Frankel’s [15] method. A 50 mg of tissue was homogenized in 1 ml chilled sucrose (0.25 M) in ice bath and centrifuged at 3,000 g for 15 min at -40°C. Supernatant was used as enzyme source. To 0.1 ml enzyme source, 0.5 ml GPT substrate and 0.5 ml 2, 4-dinitrophenylhydrazine solution was added and the mixture was incubated for 15 min at room temperature. Now, 5 ml NaOH (0.4 M) was added, mixed and incubated at room temperature for 20 min. The absorbance of mixture was measured at 505 nm. The enzyme activity has been expressed in units of GPT activity h-1 mg-1 protein.
Statistical analysis
Results were expressed as mean ± SE of six replicates. Student’s t-test was used to detect significant changes [16].
Results
Toxicity determination
The median lethal dose (LD50) of H. fastigiousus venom was 18.6 mg kg-1 body weight.
Effect of H. fastigiousus venom on hematological activity
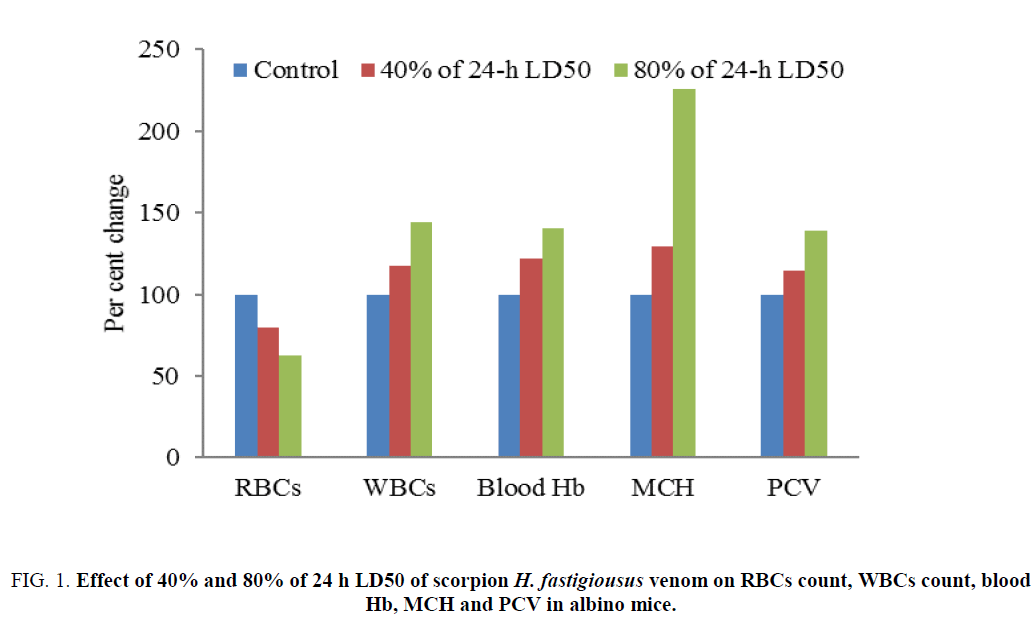
RBC count was decreased to 79.35% and 62.38% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 1). Blood hemoglobin level was increased to 122.12% and 140.71% of the controlafter 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 1). MCH level was increased to 129.63% and 225.52% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 1). Increase in WBC count was 117.19% and 144.31% of control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom (Table 1). PCV was increased to 114.38% and 138.91% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 1). Hemoglobin level in plasma was increased to 0.7 and 1.6 g 100 ml-1 of plasma after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Figure 1). The variations in RBCs count, WBCs count, blood hemoglobin, MCH, PCV and plasma hemoglobin were dose-dependent (p<0.05, Student t-test).
Figure 1: Effect of 40% and 80% of 24 h LD50 of scorpion H. fastigiousus venom on RBCs count, WBCs count, blood Hb, MCH and PCV in albino mice.
| Parameters | Control | 40% of 24 h LD50 | 80% of 24 h LD50 |
|---|---|---|---|
| RBCs | 6.54 ± 0.12 (100) |
5.19 ± 0.17 (79.35) |
4.08 ± 0.23 (62.38) |
| WBCs | 4.13 ± 0.008 (100) |
4.84 ± 0.06 (117.19) |
5.96 ± 0.09 (144.31) |
| Blood Hb* | 11.30 ± 0.14 (100) |
13.80 ± 0.16 (122.12) |
15.90 ± 0.24 (140.71) |
| MCH | 17.28 ± 0.05 (100) |
26.58 ± 0.17 (129.63) |
38.97 ± 0.25 (225.52) |
| PCV | 42.40 ± 1.14 (100) |
48.50 ± 1.13 (114.38) |
58.90 ± 1.17 (138.91) |
| Plasma Hb* | 0.00 ± 0.00 | 0.7 ± 0.16 | 1.60 ± 0.18 |
Results were expressed as mean ± SE
Values in parentheses indicate per cent change with respect to control taken as 100%.
*Values have been represented as gm 100 ml-1
Table 1: Effect of 40% and 80% of 24 h LD50 of scorpion H. fastigiousus venom on RBCs count, WBCs count, blood Hb, MCH, PCV and plasma Hb in albino mice.
Effect of H. fastigiousus venom on liver enzyme activity
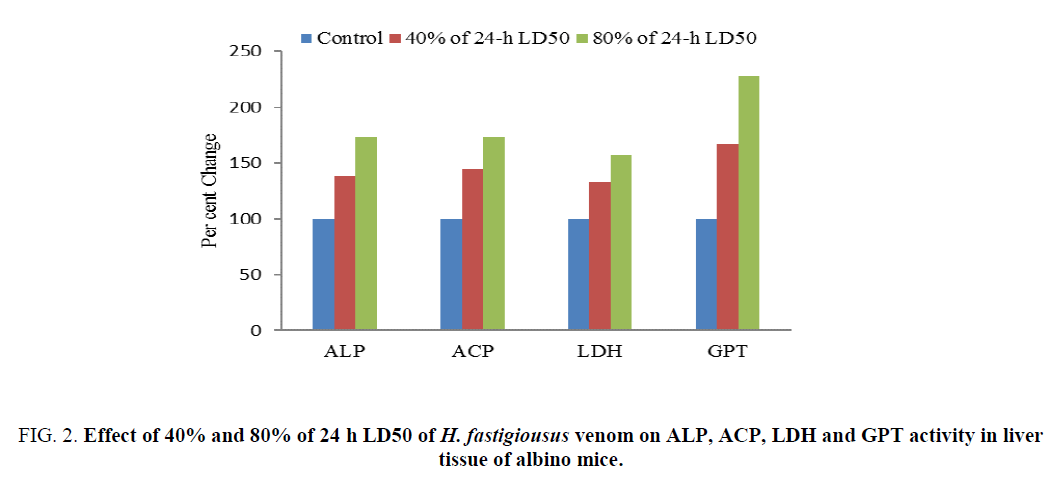
The increase in ALP activity was 138.37% and 173.25% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 2). ACP activity was increased to 144.81% and 173.71% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of H. fastigiousus venom respectively (Table 1). The increase in LDH activity was 132.56% and 157.15% of the control after 8 h of treatment with 40% and 80% of 24 h LD50 of scorpion H. fastigiousus venom respectively (Table 2). GPT activity was increased to 162.75% and 228.54% of the control after 8 h oftreatment with 40% and 80% of 24 h LD50 of scorpion H. fastigiousus venom respectively (Figure 2). The variations in the activity of these enzymes were dose-dependent (p<0.05, Student’s t-test).
Figure 2: Effect of 40% and 80% of 24 h LD50 of H. fastigiousus venom on ALP, ACP, LDH and GPT activity in liver tissue of albino mice.
| Enzymes | Control | 40% of 24 h LD50 | 80% of 24 h LD50 |
|---|---|---|---|
| ALP* | 1.72 ± 0.09 | 2.38 ± 0.13 | 2.98 ± 0.17 |
| (100) | (138.37) | (173.25) | |
| ACP* | 3.81 ± 0.16 | 5.67 ± 0.17 | 6.74 ± 0.23 |
| (100) | (144.81) | (173.71) | |
| LDH** | 508.66 ± 5.13 | 674.32 ± 6.73 | 799.31 ± 8.79 |
| (100) | (132.56) | (157.15) | |
| GPT*** | 84.74 ± 2.84 | 141.79 ± 2.53 | 193.67 ± 2.88 |
| (100) | (167.25) | (228.54) |
Results were expressed as mean ± SE
Values in parentheses indicate per cent change with respect to control taken as 100%.
* ALP and ACP enzyme activity: μ moles of p-nitro phenol formed 30 min-1 mg-1 protein
**LDH: micromoles of reduced pyruvate 45 min-1 mg-1 of protein
*** GPT: units of GPT activity h-1 mg-1 protein
Table 2: Effect of 40% and 80% of 24 h LD50 of H. fastigiousus venom on ALP, ACP, LDH and GPT activity in liver tissue of albino mice.
Discussion
H. fastigiousus venom reduced red blood cell count and increased white blood cell count, blood hemoglobin; meancorpuscular hemoglobin, packed cell volume and plasma hemoglobin in experimentally envenomed mice. Decreased red blood cell count due to the hemolytic effect of scorpion venom is supported by increased hemoglobin level in plasma [13]. This results in anemia and circulatory hypoxia [12]. Increase in blood hemoglobin after H. fastigiousus envenomation may probably be the result of hemoconcentration by massive release of catecholamines and angiotensin II [14-19]. Increase in plasma hemoglobin after H. fastigiousus envenomation indicates intravascular hemolysis [17]. When the hemolysis rate is high, the plasma extra corpuscular hemoglobin cannot be converted into bilirubin as quickly as it is released. When plasma hemoglobin concentration exceeds the hemoglobin binding capacity and kidney tubular capacity, the excess free plasma hemoglobin is filtered and excreted in the urine causing hemoglobinuria. Different scientific groups have given different reasons of intravascular hemolysis. Cobra venom releases an enzyme, phospholipase, which converts lecithin to lysolecithin, a powerful hemolytic and cytotoxic substance [17]. Since lecithin is present in red blood cells, the introduction of the venom into the body stimulates the production of hemolytic substance, lysolecithin.
This could be the cause of hemolysis associated with scorpion envenoming [20]. Hemolytic activity of scorpion venom peptides may also be associated with certain structural characteristics formed by the constituent peptides when come in contact with biological membranes [21]. An imbalance is created due to increased secretion of catabolic counter regulatory hormones like catecholamines, epinephrine, norepinephrine, glucagon, cortisol, thyroxine, triiodothyronine and reduction in anabolic hormone, insulin which might have contributed the fragility of red blood cells resulting in hemolysis [22]. Increased mean corpuscular hemoglobin after H. fastigiousus envenomation is an indicative of hemolysis [12]. Similarly, increase in white blood cells count was also reported in Mesobuthus tamulus envenomation which may probably due to the myocardial infarction [22]. Increased packed cell volume after H. fastigiousus envenomation in mice is similar to red scorpion envenomation [22]. The elevation of angiotensin II during scorpion decreases blood volume by shifting the fluid from intravascular to extravascular compartments and consequently increases packed cell volume [19,23].
ALP is a group of membrane bound enzymes which mediate transport of metabolites across the membrane and play an important role in protein and certain enzyme synthesis [24,25]. Increased ALP activity stimulates the pace of protein synthesis in liver which may probably be responsible for elevated serum protein. ACP is a lysosomal enzyme which plays an important role in catabolism, pathological necrosis, autolysis and phagocytosis [26]. Liver ischemia and hypoxia increase the activity of plasma lysosomal enzymes [27]. The increased activity of ACP might probably induce tissue necrosis and increased serum level. Tissue LDH utilizes glucose for energy especially in anaerobic conditions. Increased LDH activity occurs in response to insufficient supply of oxygen suggesting the increased glycolytic activity for obtaining energy in oxygen deficient condition [28]. The increased LDH activity in liver tissue of envenomed mice probably indicates increased glucose utilization under oxygen deficient condition. GPT works as a link between carbohydrate and protein metabolism by catalyzing the conversion of alanine to pyruvate [29]. Increased liver GPT activity is the result of stress caused by scorpion venom as stress is known to increase GPT activity [30]. During stress, energy requirement is too high to recover and glycogen level decreases. To maintain this energy requirement and to make up high decrease in glycogen level, amino acids take an active role and act as precursor of carbohydrate metabolism through transamination reaction [28].
Conclusion
Heterometrus fastigiousus venom increases ALP, ACP, LDH and GPT activity in liver tissue of albino mice. This venomreduced RBC count and increased WBC count, blood hemoglobin, MCH, PCV and plasma hemoglobin. The outcomes of this study help to understand the mechanism of asian black scorpion, H. fastigiousus venom toxicity. This will help the pharmacologists to design drugs for the treatment of accidental H. fastigiousus envenomation.
References
- Keegan HL. Scorpions of medical importance. Mississippi: University Press of Mississippi. 1986; 140.
- Gordon D, Maskowitz H, Eitan M, et al. Localization of receptor sites for insect selective toxins on Na+ channels by site directed antibodies. Biochemistry. 1992;31:7622-28.
- Bhaskaran NR, Kurup PA. Investigation on the venom of the south Indian scorpion Heterometrus scaber. Ciochem Biophys Acta. 1975;381:164-74.
- Ismail MA, El MF, Osman OH. Pharmacological studies with scorpion (Palmaneus gravimanus) venom: Evidences for the presence of histamine. Toxicon. 1975;13:49-56.
- Narayan RBS, Maniraj BL, Babu KS. Impact of scorpion Heterometrus fulvipes venom on the cholinesterase rhythmicity in the tropical mouse Mus booduga. Indian J Physiol Pharmacol. 1984;28:47-52.
- Dasgupta SC, Gomes A, Babu A, et al. Isolation, purification and immunological evaluation of toxin Hb from scorpion Heterometrus bengalensis (C.L. Koach) venom. Indian J Exp Biol. 1990;28:44-8.
- Gwee MC, Wang PT, Gopalkrishnakone P, et al. The black scorpion Heterometrus longimanus: Pharmacological and biochemical investigations of the venom. Toxicon. 1993;31:1305-1324.
- Nirthanan S, Joseph JS, Gopalkrisnakone P, et al. Biochemical and pharmacological characterization of the venom of the black scorpion (Heterometrus spinifer). Biochem Pharmacol. 2002;63:49-55.
- More SS, Kiran KM, Gadag JR. Dose dependent serum biochemical alterations in wistar albino rats after Palamneus gravimanus (Indian black scorpion) envenomation. J Basic Clin Physiol Pharmacol. 2004;15:263-75.
- Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with phenol reagent. J Biol Chem. 1951; 193:265-75.
- Kankonkar RC, Kulkurni DG, Hulikavi CB. Preparation of potent anti-scorpion-venom-serum against the venom of red scorpion (Buthus tamulus). J Postgrad Med. 1998;44:85-92.
- Dacie JV, Lewis SM. Practical Hematology. Churchill Livingstone. 1984;202-453.
- Bergmayer UH. Methods of enzymatic analysis. Academic Press.1967.
- Annon TM. Sigma diagnostic: Lactate dehydrogenase (quantitative, colorimetric determination in serum, urine and cerebrospinal fluid) at 400-500 nm. Procedure. 1985;500.
- Reitman A, Frankel SA. Colorimetric method for the determination of serum glutamate-oxaloacetate and glutamate-pyruvate transaminase. Am J Clin Pathol. 1957;28:56-63.
- Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Oxford: Blackwell Science. 2002; 817.
- Cronkite EP. Blood and Lymph. Willians and Wilkins, Baltimore. 1973;4-57.
- Goyffon M, Vachon M, Broglion N. Epidemicological clinical characteristics of the scorpion envenomation in Tunisia. Toxicon. 1982; 20:337-44.
- Murthy KRK, Vakil AE. Elevation of plasma angiotensin level in dogs by Indian red scorpion (Buthus tamulus) venom and its reversal by administration of insulin and tolazoline. Ind J Med Res. 1988;88:376-79.
- Radmanesh M. Clinical study of Hemiscorpion lepturus in Iran. J Trop Med Hyg. 1990;93:327-32.
- Torres-Larios A, Gurrola GB, Zamudio FZ, et al. a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur J Biochem. 2000;267:5023-31.
- Murthy KRK, Zare A. The use of antivenin reverses hematological and osmotic fragility changes of erythrocytes caused by Indian red scorpion Mesobuthus tamulus concanesis, Pocock in experimental envenoming. J Venom Anim Toxins. 2001;7:113-38.
- Douglas WW. Polypeptides: Angiotensin, plasma kinins and others. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The pharmacological basis of therapeutics. New York: Macmillan Publishing Company. 1985;663-76.
- Vorbrodt A. The role of phosphatase in intracellular metabolism. Posttherap Hig Med Disw. 1959;13:200-6.
- Pilo B, Asnani MV, Shah RV. Studies on wound healing and repair in pigeon liver II: Histochemical studies on acid and alkaline phosphatase during the process. J Anim Morphol Physiol. 1972;19:205-12.
- Abraham R, Goldberg L, Grasso P. Hepatic response to lysosomal effects of hypoxia, neutral red and chloroquin. Nature. 1967;215:194-6.
- Fredlund S, Ockerman PA, Vang JO. Acidosis and increased plasma levels of β-D glucosidase and β-D galactosidase after hepatic inflow occlusion in the pig. Acta Chir Scand. 1974;140:134-41.
- Lehninger AL, Cox MM, Nelson DL. Principles of Biochemistry. Worth Publisher. 2000;542:633.
- Feling P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:938.
- Knox WE, Greegard O. An introduction to enzyme physiology. Pergamon Press.1965;3:247.