Original Article
, Volume: 16( 15)Development of a Validated Stability Indicating RP-HPLC Method for Simultaneous Determination of Benazepril, Benazeprilat, and Amlodipine in Presence of Their Degradation Products and Application to Pharmaceutical Formulation and Human Plasma
- *Correspondence:
- Hemdan A, , Pharmaceutical Analytical Chemistry Department, Faculty of Pharmacy, Ahram Canadian University, 6th of October, Egypt, Tel: 201221620730; Fax: 2(02)38334379; E-mail: hemmdan@yahoo.com
Received: July 01, 2016; Accepted: August 27, 2016; Published: August 31, 2016
Citation: Hemdan A, Omar AE, Farouk M, et al. Development of a Validated Stability Indicating RP-HPLC Method for Simultaneous Determination of Benazepril, Benazeprilat, and Amlodipine in Presence of Their Degradation Products and Application to Pharmaceutical Formulation and Human Plasma. Anal Chem Ind J. 2016;16(15):112.
Abstract
A sensitive, reproducible, and rapid stability indicating RP-HPLC method was developed and subsequently validated for simultaneous determination of both benazepril (BENZ), benazeprilat (BENZT), and amlodipine (AML) in presence of their basic hydrolysis degradation products in bulk powder, pharmaceutical formulation, and application to human plasma using moexipril (MOX) as an internal standard (IS). The method uses Inertsil C18 column (250 mm × 4.6 mm, 5 μ) and the mobile phase used was consisting of acetonitrile-potassium hydrogen phosphate buffer pH 7 adjusted by 0.1 N NaOH (40/60 v/v) with a flow rate of 1.0 mL/min and detection at 242 nm. A detailed validation of the methods was performed following the ICH guidelines and the standard curves were found to be linear in the range of 0.5 μg/mL to 100 μg/mL, 0.5 μg/mL to 100 μg/mL and 5 μg/mL to 100 μg/mL for BENZ, BENZT, and AML, respectively. Statistical comparison was done between the proposed method and the reported one where no significant difference was found between the two methods.
Keywords
Benazepril; Benazeprilat; Amlodipine; Stability indicating; RP-HPLC
Introduction
Benazepril, (3S)-3-[(1S)-1-ethoxycarbonyl-3 phenylpropylamino]-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepin-1-yl] acetic acid hydrochloride is an antihypertensive drug, which belongs to the group of angiotensin convertase inhibitors. It acts on the renin-angiotensin-aldosterone system by inhibition of the conversion of the inactive angiotensin I to the highly potent vasoconstrictor angiotensin II. It also reduces the degradation of bradykinin. Benazepril HCl is applied in pharmacotherapy as a first choice drug for treatment of arterial hypertension, ischemic heart disease, hypertrophy of the left heart ventricle and post infarction heart dysfunction [1]. In vivo, benazepril is hydrolyzed to a pharmacologically active metabolite, the diacid benazeprilat, (3-[(1-carbonyl-3-phenyl-(1S)-propyl)-amino]-2,3,4,-5-tetrahydro-2-oxo-1-(3S)-benazepine-1-acetic acid). Amlodipine is a potent dihydropyridine calcium antagonist [2,3] useful in the management of angina pectoris and hypertension [4].
It is reported that benazepril was identified by HPLC [5-8], spectrophotometry [9-15], and with benazeprilat by liquid chromatography-mass spectrometry [16,17], and gas chromatography-mass spectrometry [18]. Also it is reported that amlodipine was determined by HPLC [19,20] spectrophotometry [21,22]. Benazepril with Amlodipine also were determined by HPLC [23], spectrophotometry [24], and liquid chromatography-mass spectrometry [25]. Loadless®, a drug used in the Egyptian market, which is a combination of BENZ and AML is used for the treatment of hypertension. Literature survey revealed that BENZ is approved in United States Pharmacopeia [26], while AML is approved in British Pharmacopeia [27]. Only one of the reported methods [6] can be comparable to the present one, but the run time was about 20 min. and the sensitivity was intermediate, also there is no quantitation of the active metabolite BENZT. Our goal in this manuscript is to shorten the run time and increase the method sensitivity to be applicable to determination of BENZ, BENZT, and AML in biological fluids as human plasma. Moreover, there is no structure elucidation of the degradation products in the reported method [6], where our present study introduces structure elucidation of the basic degradation products.
Experimental
Material and reagents
BENZ, AML, and MOX (IS) were kindly supplied from National Organization for Drug Control and Research (NODCAR, Cairo, Egypt). The purity of the standards was certified to be higher than 99%. Structures of the compounds are shown in Figure. 1.
Loadless®commercial capsules, labeled to contain 5 mg AML and 20 mg BENZ batch number 520034, 520047, 520080 was obtained from local market.
• Methanol: HPLC-grade (Sigma-Aldrich).
• Acetonitrile: HPLC-grade (Sigma-Aldrich).
• Double distilled water was used.
• Other reagents were of analytical-grade.
Instrument
Analysis was performed on a chromatographic system Jasco LC-Net II/ADC (Japan) equipped with UV detector (UV-2070 plus), isocratic pump (PU-2080 plus) and 4-line degasser (DG-2080-54). A chromatographic separation was achieved by Inertsil C-18, 250 mm × 4.6 mm, 5 μ analytical column. Data acquisition were made with Chrom NAV software.
Mass spectrometer for structure elucidation of the degradation product
This is done by MS/MS detection in positive ion mode using a MDS Sciex (Foster City, CA, USA) API-3200 MS/MS triple quadrupole mass spectrometer, equipped with a Turbo ion spray interface at 350°C. The common parameters, nebulizer gas (GS1), heater gas (GS2) and collision activated dissociation gas (CAD), were set at 30, 40, and 5 psi, respectively. The compound parameters, declustering potential (DP), collision energy (CE) and collision exit potential (CXP) were set at 56, 31, and 12 V respectively. The analytical data were processed using Analyst software (version 1.4.2).
Procedure
Preparation of the basic degradation products of the studied drugs
BENZ and AML were subjected to basic degradation. 100 mg of each powder was weighed, transferred separately to two conical flasks. Then, 0.1 N NaOH was added separately on the two volumetric flasks. Then the flasks were refluxed for 30 min for basic degradation. After the reflux, the conical flasks were neutralized against 0.1 N HCl, and then completed to 100 mL by distilled water. The complete degradation was confirmed using HPLC and mass spectrometry.
Preparation of the acidic degradation product of BENZ
BENZ was weighed for 100 mg and transferred to a conical flask. Then, 0.1 M HCl was added, and the flask was refluxed at 90°C for 3 h. After the reflux, the content was neutralized against 0.1 M NaOH, transferred quantitatively to 100 mL volumetric flask, and then completed to 100 mL by distilled water.
Chromatographic conditions
Separation of the two analytes along with the degradation products and the IS was accomplished using Inertsil C18 column (250 mm × 4.6 mm, 5 μ column) as a stationary phase. The mobile phase used was acetonitrile-potassium hydrogen phosphate buffer pH=7 adjusted by 0.1 N NaOH (40/60 v/v) was used as a mobile phase. The flow rate was 1 mL/min and the detection wavelength was 242 nm.
Preparation of standard stock and working solutions
Primary stock solutions of BENZ, AML, and MOX internal standard (IS) (all at 1.0 mg/mL) were separately prepared by dissolving 100 mg of each standard powder in the least amount of methanol and completed to the volume by distilled water, where BENZT was prepared by degradation (as explained earlier). Primary stock solutions were diluted with the mobile phase for the RP-HPLC to prepare standard working solutions of BENZ, BENZT, AML, and MOX (100 μg/mL). All solutions were stored at 4°C, and equilibrated to room temperature before use.
Linearity and calibration standards of the pure bulk powder
Accurately measured aliquots of BENZ, BENZT, and AML were transferred from their working standard solution (0.1 mg/mL) into three series of 10 ml volumetric flasks and complete to volume with the mobile phase (acetonitrile-potassium hydrogen phosphate buffer (40/60 v/v) pH=7 adjusted by 0.1 N NaOH). The calibration samples consist of six concentrations of BENZ (0.5 μg/mL to 100 μg/mL), BENZT (0.5 μg/mL to 100 μg/mL), and AML (5 μg/mL to 100 μg/mL). The samples were injected separately along with MOX (IS) into the Inertsil C18 column under a flow rate of 1 mL/min. The relative peak area of each analyte was recorded against its concentration, the linearity curves were constructed and the regression equations computed.
Validation
Accuracy: The accuracy of the results was checked by applying the proposed methods for determination of three replicates of different concentrations of the analytes. The concentrations were obtained from the corresponding regression equations, from which the percentage recoveries suggested good accuracy of the proposed methods.
Precision
Repeatability: Three concentrations of the analytes were analyzed three times intra-daily using the proposed methods under the same experimental conditions. The relative standard deviations were calculated.
Reproducibility (intermediate precision): The previous procedures were repeated inter-daily on three different days for the analysis of the three chosen concentrations. The relative standard deviations were calculated.
Laboratory prepared mixtures (selectivity): Solutions containing different ratios of the analytes were prepared by transferring accurately measured aliquots from their standard working solutions into a series of 10 ml volumetric flasks and the volume was completed to the mark with mobile phase. The final concentration ranges were 5 μg/mL to 20 μg/mL for BENZ, 10 μg/mL to 25 μg/mL for BENZT, and 5 μg/mL to 25 μg/mL for AML. The chromatograms of these different laboratory prepared mixtures were recorded and the procedure under linearity was then followed. Concentrations of the analytes in the prepared samples were calculated from the corresponding computed regression equations.
Application to pharmaceutical formulation
To determine the content of BENZ and AML in commercial capsules (Loadless®) (each capsule labeled to contain 20 mg BENZ and 5 mg AML), 20 capsules were weighed and finely powdered. A portion of powder equivalent to one capsule was weighed accurately and transferred to a 100 ml beaker. Methanol (50 ml) was added, stirred using a magnetic stirrer for 15 min and filtered through 0.5 μm Whatman filter paper into a 100 ml volumetric flask. The residue was washed three times each with 10 ml of methanol and the solution was completed to the mark with the same solvent. From the above prepared solution, further dilutions were prepared in the obtained linearity ranges using mobile phase. The general procedure described under linearity was followed to determine the concentration of both drugs in the prepared dosage form solution. The analysis was done in triplicates. Concentrations of BENZ and AML in the prepared samples were calculated from the corresponding computed regression equations.
Application to spiked human plasma sample
Aliquot volumes from the standard working solutions of BENZ, BENZT, and AML were added on 500 μL human plasma, then 30 μL IS was added. Then, 3 mL of acetonitrile was added, the samples were mixed on a vortex for 1 min, followed by centrifugation for 10 min at 10,000 rpm. The organic phase solution was transferred to a clean tube and evaporated to dryness under a gentle stream of nitrogen gas at 40°C. The residue was reconstituted with 100 μL mobile phase, and 20 μL was injected. Then, the same procedure was repeated but spiking was done after the extraction step to calculate the recovery percentage.
Results and Discussion
This manuscript describes the use of a RP-HPLC method to quantify BENZ, its active metabolite BENZT, and AML in the pharmaceutical dosage form. Also the paper describes the application of the proposed method to determine the three analytes in spiked human plasma without interference form endogenous plasma constituents. All the determinations were done in a short run time and with high sensitivity. The novelty in our work is the analysis and quantitation of BENZ, AML, and the active degradation product of BENZ which is BENZT. BENZT is the active moiety that exerts the antihypertensive effect in the body, so its quantitation is very important. Also, full structure elucidation of the analytes and their degradation products was done using tandem mass spectrometry.
Method development
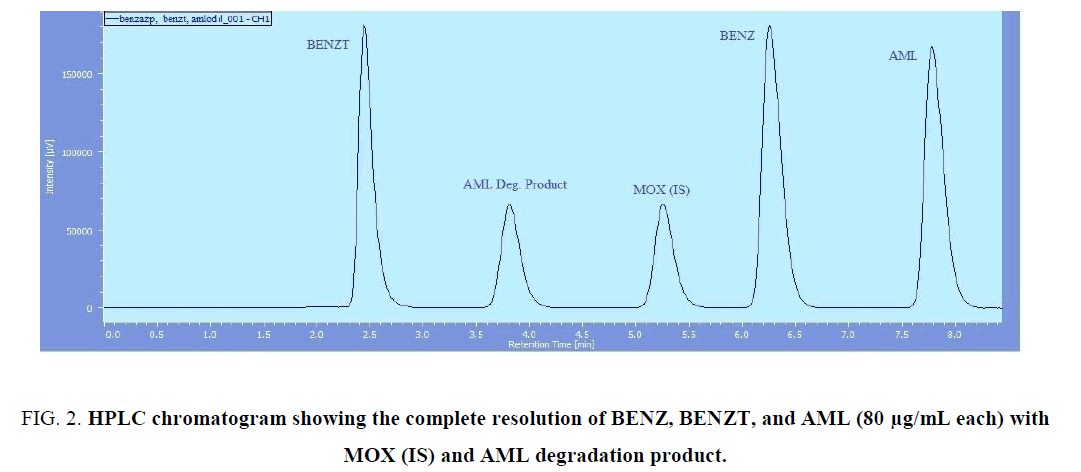
Different organic modifiers proportions along with different buffers with different pH were tried. Our goal is to obtain optimum resolution, symmetric peak shape, reasonable run time, and best sensitivity Table 1. Methanol and acetonitrile were tried, methanol caused longer run time where the run time extended to more than 20 min. Phosphate buffer with different pH’s (3, 5, 7) were tried. The optimum resolution and peak shape obtained with acetonitrile-potassium hydrogen phosphate buffer pH=7 adjusted by 0.1 N NaOH (40/60 v/v) as a mobile phase. The flow rate for better resolution and rapid separation was adjusted to 1 mL/min. Also, two types of stationary phases C8 and C18 were investigated for the optimum resolution of the analytes’ peaks, however the more hydrophobic Inertsil C18 was found to be more superior in separating analytes’ peaks in a reasonable run time. All the tried conditions are summarized in Table 1. Full separation of the three peaks of BENZ, BENZT, and AML was obtained, where the retention time of the analytes was 6.3 min, 2.4 min, and 7.8 min, respectively, where the retention time for MOX (IS) was 5.3 min as shown in (Figure 1). System suitability parameters are shown in Table 2.
Figure 2: HPLC chromatogram showing the complete resolution of BENZ, BENZT, and AML (80 μg/mL each) with MOX (IS) and AML degradation product.
| Organic modifier % | Result |
|---|---|
| 50% Methanol | Long run time more than 15 min |
| 40% Methanol | Long run time more than 20 min |
| 50% Acetonitrile | Bad resolution |
| 40% Acetonitrile | Good resolution |
| pH | Result |
| 3 | Bad resolution for degradation and Amlodipine peaks |
| 5 | Bad peak shape |
| 7 | Ideal resolution and peak shape |
Table 1: Different conditions tried for obtaining good separation and peak shape.
| Parameter | Benazepril | Benzeprilat | Amlodipine |
|---|---|---|---|
| Retention time | 6.3 | 2.4 | 7.8 |
| Resolution* | 3.5 | 4.2 | - |
| Tailing factor | 1.2 | 1.2 | 1.3 |
| Column Capacity | 4.73 | 1.18 | 6.09 |
| Column efficiency(No. of theoretical plates) | 6879 | 1955 | 6348 |
| HETP | 0.004 | 0.013 | 0.004 |
*Resolution is calculated relative to the next peak.
Table 2: System suitability parameters of the proposed RP-HPLC method.
Stability of BENZ and AML
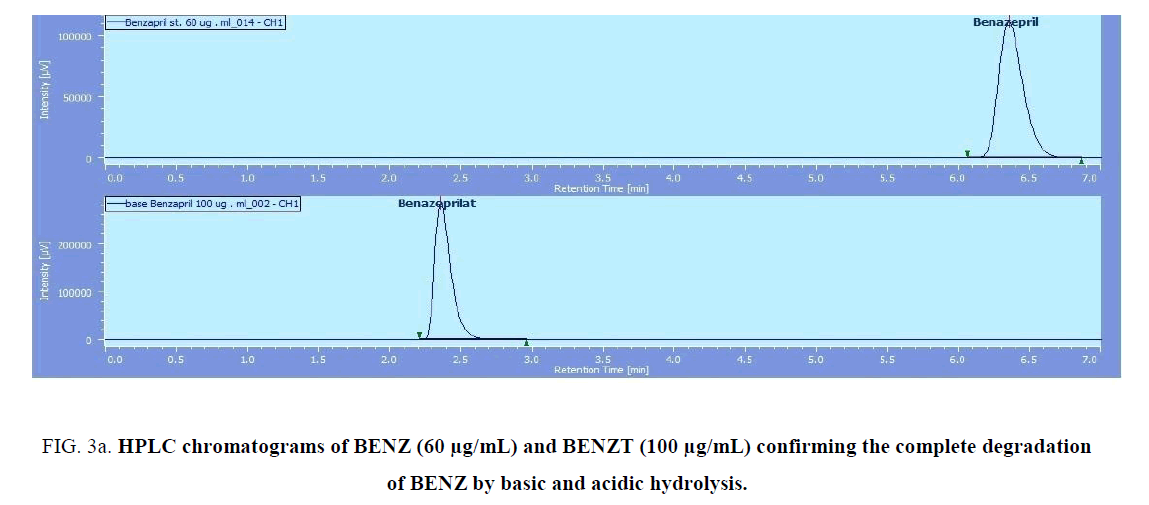
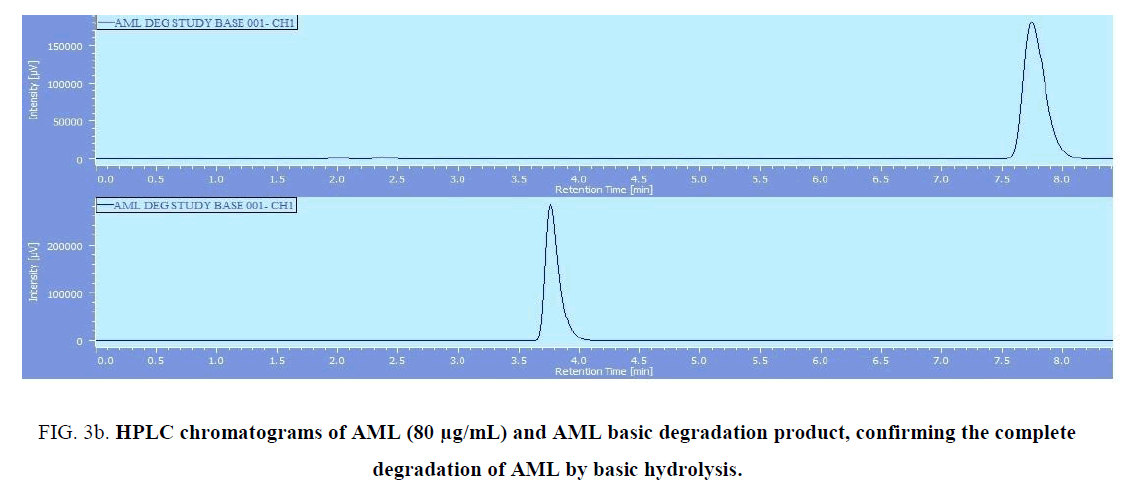
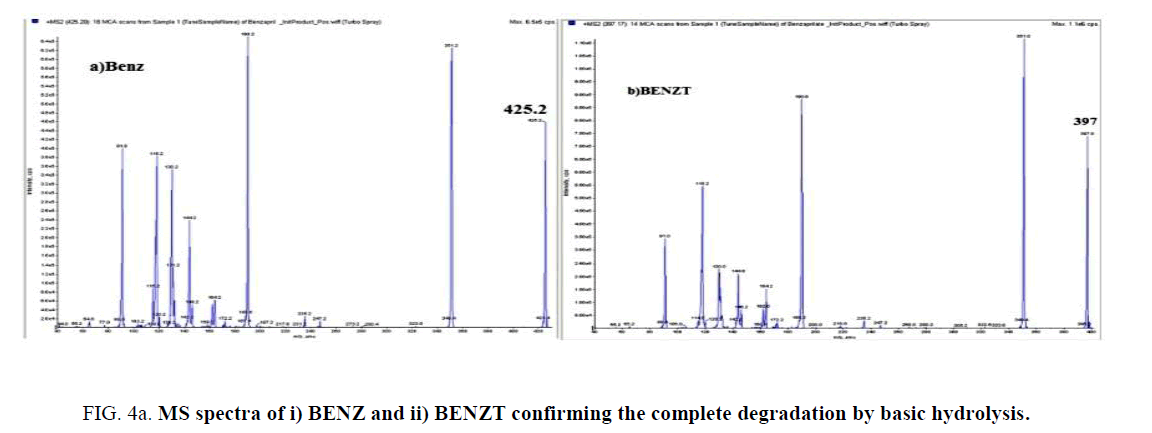
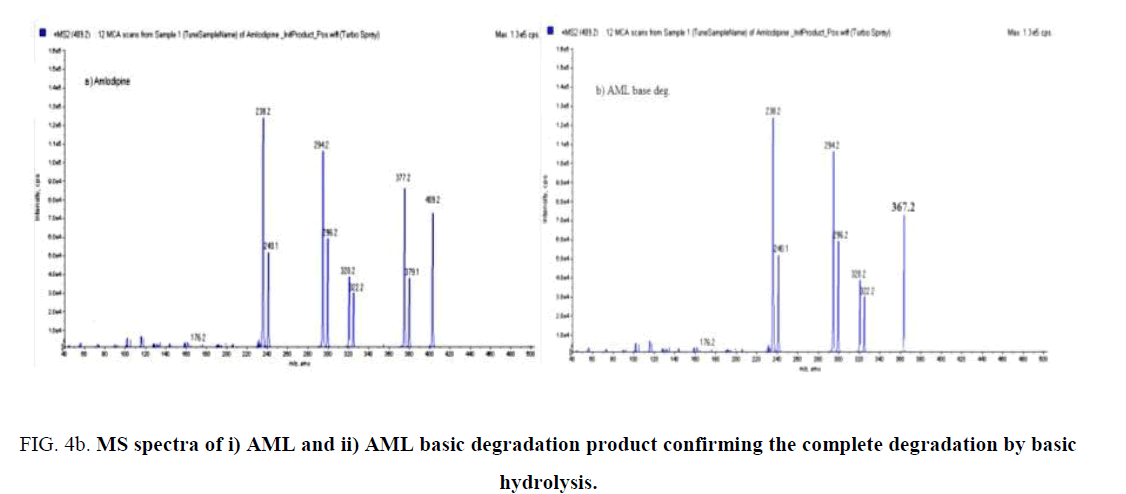
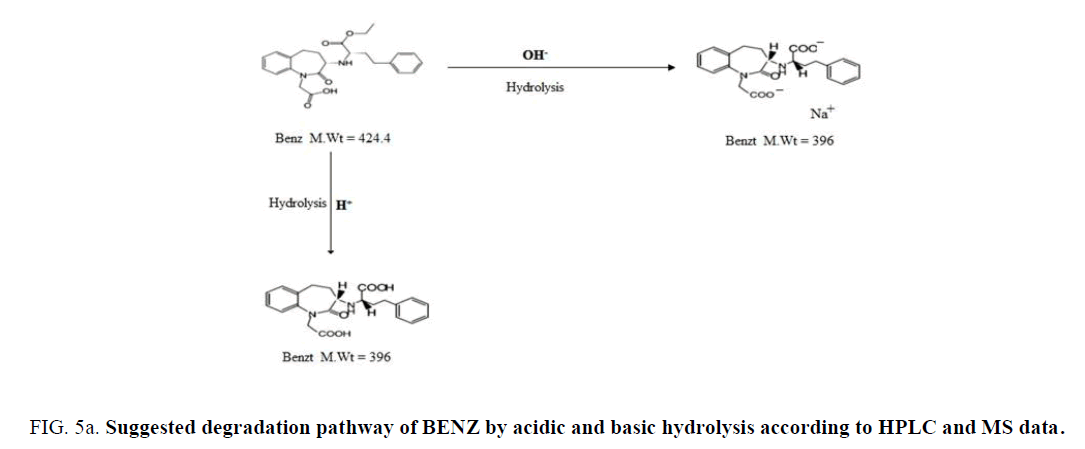
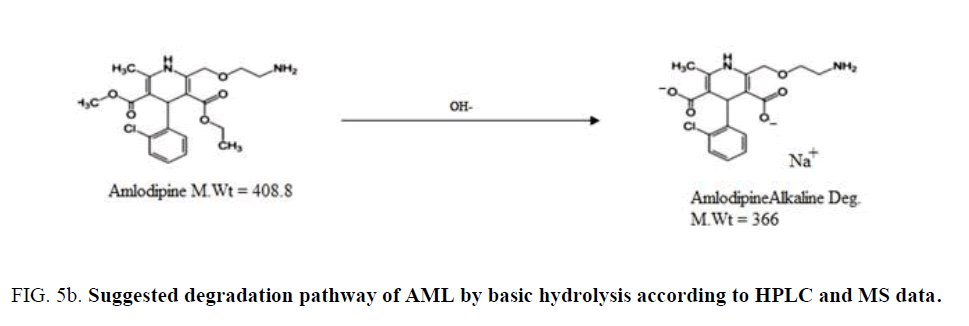
BENZ with a molecular weight (424.5) is an ester pro-drug which is hydrolyzed in vivo by esterase enzyme to the pharmacologically active biacid BENZT with a molecular weight (396). So to obtain this active form, hydrolysis of BENZ was tried by exposing to 0.1 N NaOH and refluxing for 30 min for basic degradation. Also complete degradation of AML was done by the same method. AML has a molecular weight of (408.8) where its basic degradation has a molecular weight (366). The complete degradation was confirmed by HPLC (with the disappearance of BENZ and AML peaks at retention times 6.3 min and 7.8 min, respectively. and appearance of the BENZT and basic degradation product of AML peaks at retention times 2.4 min and 3.8 min, respectively ((Figure 3a) and (Figure 3b)), and mass spectrometry (with the appearance of m/z for BENZT and degradation product of AML at 397 and 367.2, respectively, and disappearance of m/z for BENZ and AML at 425.2 and 409.2, respectively) as shown in (Figure 4a)and (Figure 4b). The structures of the degradation products were suggested according to HPLC and MS data as shown in (Figure 5a) and (Figure 5b).
Figure 3a: HPLC chromatograms of BENZ (60 μg/mL) and BENZT (100 μg/mL) confirming the complete degradation of BENZ by basic and acidic hydrolysis.
Figure 3b: HPLC chromatograms of AML (80 μg/mL) and AML basic degradation product, confirming the complete degradation of AML by basic hydrolysis.
Figure 4a: MS spectra of i) BENZ and ii) BENZT confirming the complete degradation by basic hydrolysis.
Figure 4b: MS spectra of i) AML and ii) AML basic degradation product confirming the complete degradation by basic hydrolysis.
Figure 5a: Suggested degradation pathway of BENZ by acidic and basic hydrolysis according to HPLC and MS data.
Validation
Validation of the proposed methods was assessed according to ICH guidelines [28].
Linearity and Range: The linearity and range of the method were evaluated by analyzing six concentrations of BENZ, BENZT, and AML. Each concentration was repeated three times. The assay was performed according to the experimental conditions previously mentioned. The linear regression equations are summarized in Table 3.
| Parameter | Benazepril | Benazeprilat | Amlodipine |
|---|---|---|---|
| Range µg/mL | 0.5-100 | 0.5-100 | 5-100 |
| Regression Equation | y=0.0454x+0.0222 | y=0.0423x+0.0142 | y=0.0572x+0.0577 |
| Correlation coefficient (r) | 0.9998 | 0.9998 | 0.9998 |
| Accuracya | 99.54 ± 1.045 | 101.25± 0.854 | 100.73 ± 1.356 |
| Repeatabilityb | 98.26 ± 1.211 | 100.25± 1.341 | 99.14 ± 1.234 |
| RSD% | 1.232 | 1.338 | 1.245 |
| Intermediate precisionc | 99.12 ± 1.231 | 99.21± 1.421 | 101.21 ± 1.544 |
| RSD% | 1.242 | 1.432 | 1.526 |
aThree concentrations of each analyte (10 µg/mL, 15 µg/mL and 25 µg/mL), repeated three times for each concentration
bIntra-day (n=3), average of three concentrations of the analytes (10 µg/mL,15 µg/mL and 25 µg/mL) repeated 3 times within the same day;
cInter-day (n=3), average of three concentrations of the analytes (10 µg/mL, 15 µg/mL and 25 µg/mL) repeated 3 times in three consecutive days.
Table 3: Validation parameters of the proposed chromatographic methods.
Accuracy: The accuracy of the results was checked by applying the proposed methods for determination of different samples of BENZ, BENZT, and AML. The concentrations were obtained from the corresponding regression equations, from which the percentage recoveries suggested good accuracy of the proposed methods. Results are shown in Table 3.
Precision
Repeatability: Three concentrations of BENZ, BENZT, and AML were analyzed three times intra-daily using the proposed methods. The relative standard deviations were calculated Table 3.
Reproducibility (intermediate precision): The previous procedures were repeated inter-daily on three different days for the analysis of BENZ, BENZT, and AML. The relative standard deviations were calculated (Table 3).
Selectivity: Selectivity of the methods was achieved by the analysis of different laboratory prepared mixtures of BENZ, BENZT, and AML within the linearity range. Satisfactory results were obtained as shown in Table 4.
| Concentration (µg/mL) | (Recovery % ± SD) | ||||
|---|---|---|---|---|---|
| Benazepril | Benazeprilat | Amlodipine | Benazepril | Benazeprilat | Amlodipine |
| 20 | 10 | 5 | 99.25 ± 0.251 | 99.47 ± 0.421 | 101.25 ± 0.585 |
| 15 | 15 | 10 | 99.87 ± 0.341 | 98.41 ± 0.1214 | 99.54 ± 1.471 |
| 10 | 20 | 15 | 98.65 ± 0.428 | 99.25 ± 0.751 | 99.15 ± 0.778 |
| 5 | 25 | 20 | 98.32 ± 0.497 | 99.41 ± 0.674 | 100.45 ± 0.541 |
All Calculations were done in triplicates.
Table 4 : . Determination of the three analytes in their laboratory prepared mixtures by the proposed methods.
Application of the method in assay of capsules
The proposed method was applied for the determination of BENZ and AML in their combined pharmaceutical formulation (Loadless® capsules). The validity of the methods was assessed by applying the standard addition technique (Table 5). It shows that the developed methods are accurate and specific for determination of the cited drugs in presence of dosage form excipients.
| Loadless®Product | Claimed (μg/mL) | Standard addition | Recovery (Mean ± SD) | |||
|---|---|---|---|---|---|---|
| Added (μg/mL) | Found (μg/mL) | Recovery (%) | Proposed method | Standard addition | ||
| Benazepril | 40 | 5 | 5.05 | 101.00 | 100.45 ± 0.248 | 99.78 ± 1.086 |
| 10 | 9.89 | 98.90 | ||||
| 15 | 14.92 | 99.47 | ||||
| Amlodipine | 10 | 5 | 4.96 | 99.20 | 99.86 ± 0.485 | 99.02 ± 0.367 |
| 10 | 9.86 | 98.60 | ||||
| 15 | 14.89 | 99.27 | ||||
Table 5: Frequency of local recurrences after organ-preserving therapy in patients followed for 240 months.
Application to spiked human plasma sample
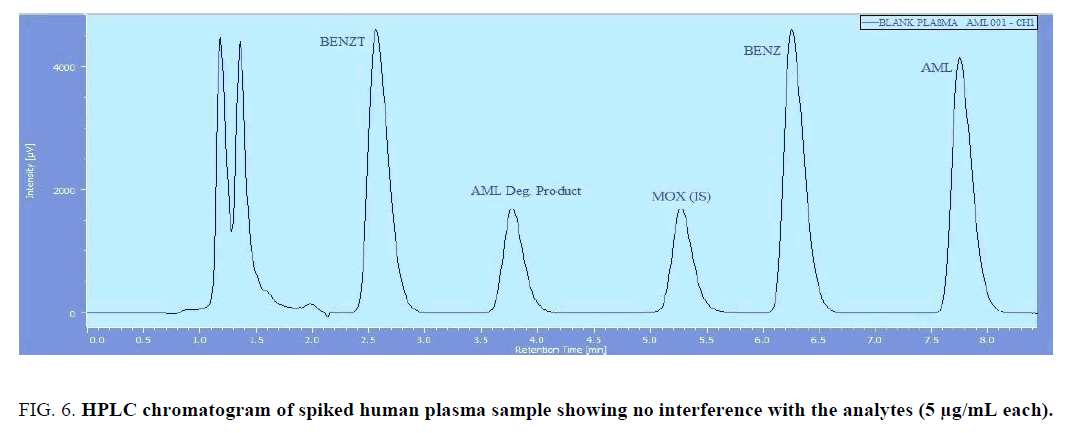
The chromatographic method was applied for the determination of BENZ, BENZT, and AML in biological fluids. Drug free human plasma was obtained from healthy volunteer, and then Spiked with BENZ, BENZT, and AML. The method showed no interference from endogenous plasma constituents as shown in (Figure 6). The recovery data are presented in Table 6.
Figure 6: HPLC chromatogram of spiked human plasma sample showing no interference with the analytes (5 µg/mL each).
| Spiked Concentration µg/mL | Recovery % ± SD* | ||||
|---|---|---|---|---|---|
| Benazepril | Benazeprilat | Amlodipine | Benazepril | Benazeprilat | Amlodipine |
| 1 | 1 | 10 | 85.47 ± 3.234 | 88.34 ± 2.715 | 80.26 ± 3.471 |
| 2 | 2 | 20 | 84.22 ± 4.724 | 88.82 ± 3.421 | 81.35 ± 3.826 |
| 5 | 5 | 30 | 84.63 ± 4.871 | 87.44 ± 2.514 | 80.64 ± 5.286 |
*The mean percentage recovery of 3-separate determinations.
Table 6: Determination of the analyte in spiked human plasma by the proposed RP-HPLC method.
Statistical analysis
Results obtained by the proposed method for the determination of pure samples of BENZ, BENZT and AML are statistically compared to those obtained by the reported methods. The calculated t and F values were found to be less than their corresponding theoretical ones confirming good accuracy and excellent precision Table 7. Separation was achieved on Zorbax SB C18, 5 μ, 250 mm × 4.6 mm i.d. column using phosphate buffer and acetonitrile in the proportion of 65:35 (v/v) with apparent pH adjusted to 7.0 at a flow rate of 1ml/min, and UV detection at 240 nm using a photodiode array detector.
| Parameter | Benazepril | Amlodipine | ||
|---|---|---|---|---|
| Reference Method | RP-HPLC | Reference Method | RP-HPLC | |
| Mean | 100.41 | 99.54 | 100.12 | 100.73 |
| SD | 0.482 | 1.045 | 0.924 | 1.356 |
| N | 6 | 6 | 6 | 6 |
| Variance | 0.232 | 1.092 | 0.853 | 1.838 |
| Student t (2.23)a | 1.325 | 1.751 | ||
| F test (5.05)a | 4.706 | 2.154 | ||
aThe values in parenthesis are the corresponding theoritical values of t and F at p=0.05.
Table 7: Statistical comparison for the results obtained by the proposed methods and the reference methods [6] for the determination of amlodipine and benazepril in pure powder form.
Stability
BENZ, BENZT, and AML working solutions showed no changes up to two weeks when stored at room temperature.
Conclusion
In summary, we developed and validated a highly sensitive, specific, reproducible and high-throughput stability indicating chromatographic methods for simultaneous quantification of BENZ, BENZT, and AML in bulk powder and pharmaceutical formulation. Also the chromatographic method was applied on spiked human plasma. According to the validation parameters, we concluded that the developed method could be useful for quality control laboratories and bioequivalent studies with desired precision and accuracy.
References
- Pratap YP, Rupali SJ, Vijay S, et al. Simultaneous spectrophotometric estimation of Amlodipine Besylate and Benazepril HCl in pure and pharmaceutical dosage form. Der PharmaLett. 2011;3:397-03.
- Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118(5):1100-03.
- Kungys G, Naujoks, H, Wanner C. Pharmacokinetics of amlodipine in hypertensive patients undergoing haemodialysis. Eur J Clin Pharmacol. 2003;59(4):291-5.
- Reid J, Meredith, P, Elliot H. Pharmacokinetics of calcium antagonists. J Cardiovasc Pharmacol. 1988;12:22-6.
- Sarat M, MuraliP, Rambabu C. Development and Validation of rp-hplc Method for Simultaneous Estimation of Amlodipine besylate and Benazepril HCl in Tablet Dosage Form. Int Curr Pharm J. 2012;4:80-4.
- Raghu N, Udhav N, Murlidhar S. Stability indicating RP-HPLC method for simultaneous determination of amlodipine and benazepril hydrochloride from their combination drug product. J Pharm Biomed Anal. 2005;39:147-55.
- Radhakrishna T, Rao D, Vyas K, et al. A validated method for the determination and purity evaluation of benazepril hydrochloride in bulk and in pharmaceutical dosage forms by liquid chromatography. J Pharm Biomed Anal. 2000;22:641-50.
- Xue DW, Eli C, Xiao C, et al. Simultaneous and rapid quantitation of benazepril and benazeprilat in human plasma by high performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal. 2007;44:224-30.
- Madhavi L, Shireesha L, Tuljarani G. Spectrophotometric estimation of Valsartan and Benazepril hydrochloride in Pure and Pharmaceutical Formulations. Int J ChemTech Res. 2011;3:1830-4.
- Belal F, Al-Zaagi IA, Abounassif MA. Spectrophotometric determination of benazepril in tablets. Farmaco. 2000;55(6-7):425-32.
- Panderi IE. Simultaneous determination of benazepril hydrochloride and hydrochlorothiazide in tablets by second-order derivative spectrophotometry. J Pharm Biomed Anal. 1999;21(2):257-65.
- El-Gindy A, Ashour A, Abdel-Fattah L, et al. Spectrophotometric determination of benazepril hydrochloride and hydrochlorothiazide in binary mixture using second derivative, second derivative of the ratio spectra and chemometric methods. J Pharm Biomed Anal. 2001;25:299-07.
- El-Yazbi F, Abdine H, Shaalan R. Spectrophotometric methods for the determination of benazepril hydrochloride in its single and multi-component dosage forms. J Pharm Biomed Anal. 1999;20:343-50.
- Durmus Ö, Erdal D. Determination of Benazepril HCl and Hydrochlorothiazide in Pharmaceutical Preparations Using UV-Visible Spectrophotometry and Genetic Multivariate Calibration Methods. J Food Drug Anal. 2005;13:301-11.
- Beata S, Sylwia P, Marcin, L. Validation of UV derivative spectrophotometric method for determination of benazepril hydrochloride in tablets and evaluation of its stability. Acta Poloniae PharmaceuticaDrug Res. 2009;66:343-9.
- Weitao X, Bo C, Shouzhuo Y, et al. Simultaneous determination of benazepril hydrochloride and benazeprilat in plasma by high performance liquid chromatography/electrospray mass spectrometry. J Chromatogr B. 2005;814:303-8.
- Chen K, Zhang J, Liu S, et al. Simultaneous determination of lercanidipine, benazepril and benazeprilat in plasma by LC-MS/MS and its application to a toxicokinetics study. J Chromatogr B AnalTechnol Biomed Life Sci. 2012;899:1-7.
- Pommier F, Boschet F, Gosset G. Quantitative determination of benazepril and benazeprilat in human plasma by gas chromatography-mass spectrometry using automated 96-well disk plate solid-phase extraction for sample preparation. J Chromatogr B. 2003;783:199-05.
- Pournima S. Harinath N, Sachin A. RP-HPLC method for simultaneous estimation of amlodipine besylate and olmesartan medoxomil from tablet. J Pharm Pharm Sci. 2011;3:146-9.
- Shah DA, Bhatt KK, Mehta RS, et al. Stability Indicating RP-HPLC Estimation of Atorvastatin Calcium and Amlodipine Besylate in Pharmaceutical Formulations. Indian J Pharm Sci. 2008;70(6):754-60.
- Vijaya V, Vrushali T, Vrushali K, et al. Spectrophotometric simultaneous determination of amlodipine besylate and hydrochlorothiazide incombined tablet dosage form by simultaneous equation, absorption ratio and first order derivative spectroscopy methods. Int J Chem Res. 2011;2:7-10.
- Devi R, Ramakrishna S. New spectrophotometric methods for simultaneous determination of amlodipine besylate and atorvastatin calcium in tablet dosage forms. J Pharm Pharm Sci. 2010;2:215-9.
- Shiva PB, Karuna PD. Quantitative determination of amlodipine in human plasma by ultra-performance liquid chromatography- electro spray ionization mass spectrometry: application to a clinical pharmacokinetic study. Asian J Pharm Clin Res. 2012;5:89-93.
- Abhi K, Kishan S, Manju M. Application of absorption correction method and first order derivative spectrophotometry for simultaneous determination of anti-hypertensive combination (amlodipine and benazepril). J Pharm Pharm Sci. 2013;5:371-5.
- Nageswara RP, Jaswanth KI, Ramesh M, et al. Simultaneous determination of atorvastatin, amlodipine, ramipril and benazepril in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. J Biomed Chromatogr. 2010;25:439-49.
- United States Pharmacopeial Convention. United States pharmacopeia (2007) 30 NF 25, Rockville.
- British Pharmacopoeia. Medicines and Healthcare Products Regulatory Agency (MHRA), London; 2007.
- ICH. Validation of analytical procedures: Text and methodology Q2 k(R1). International Conference on Harmonization; Geneva; 2005.