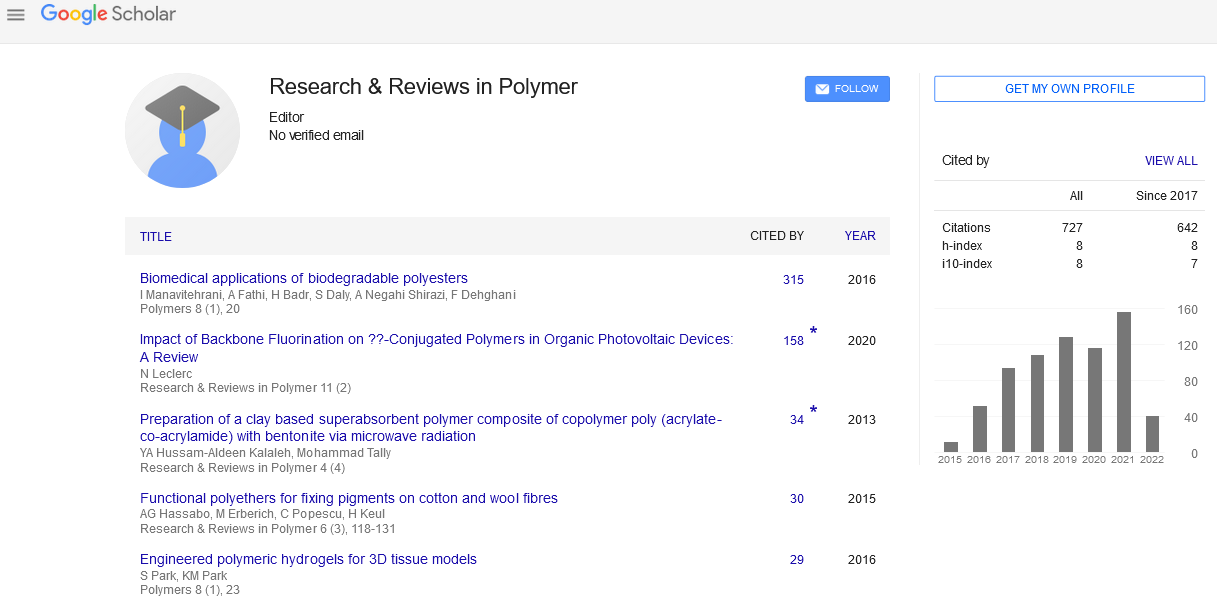
Current opinion
, Volume: 10( 1)Determination of Reactivity Ratios for ÃZ±-Methylstyrene-Butadiene Copolymerization via Cesium-based Catalyst System
Kwang Su Seo, Susan A Lettieri, Steve K Henning, Weng-Liang Hsu, Adel F Halasa*
Institute of Polymer Science, The University of Akron, Akron, OH, USA The Goodyear Tire and Rubber Co., 142 Goodyear Blvd., Akron, OH, USA
E-mail: halasa@uakron.edu
Abstract
In low temperature polymerization system, the use of α-methylstyrene as a monomer has been limited due to the ceiling temperature of α-methylstyrene. The limitation of low temperatures obtained from the thermodynamic equilibrium that supports a dimer over higher oligomers as the a-methylstyrene ceiling temperature is approached. Conjugated dienes in solvent of hydrocarbon at 65-75°C and copolymerization of α-methylstyrene was studied using a novel new catalyst system based on group I of organometallic compound (n-butyl lithium) or group II (dibutyl magnesium) in combination with the cesium of 2-etylhexyl alcohol or alkoxides potassium in the presence of chelating diamine such as, N,N,N’,N’-tetramethylethylene diamine [TMEDA]. The purpose of this research is to determine poly(α-methylstyrene-co-butadiene) polymerization reactivity ratios in cesium-based catalyst system. These data can be used to determine the characteristics incorporation order of the monomers into the polymer chain and model the chain microstructure. The catalyst system based on the TMEDA/ cesium alkoxide /dibutyl magnesium in molar ratio 2:2:1 has earlier proven that butadiene and a comonomer mixture of α-methylstyrene can be copolymerized at 65°C without the α-methylstyrene homopolymerization. The included approach and Differential techniques of determining r1 and r2 totally based on composition of copolymer equation may be used to model the poly (α-methylstyrene-co-butadiene) rubber (α-MeSBR) copolymerization processs.
The α-methylstyrene monomer should be incorporated into the polymer chain as singlet units with the opportunityof atiny low fraction of diads. In excessive temperature, the speed of self-propagation for α-methylstyrene is near zero, the cross-propagation rate must be higher. Consequently, α-methylstyrene is also completely transformed right into a polymer chain, limiting the negative rate effect of depropagation. The anionic solution polymerization of 1,3-butadiene and α-methylstyrene was polymerized at 10°C with different feed ratios within the presence of dialkyl magnesium, chelating diamine and Cesium alkoxide. The butadiene reactivity ratio at 50/50 wt% (35/65 molar ratio) α-methylstyrene/butadiene calculated as approx.1.50, while for α-methylstyrene calculated by both the integrated and differential methods were the values of 0.40 and -0.1. This research specifies that equation of copolymer composition must be modified to encompass propagation-depropagation equilibrium phenomenon.
Keywords
Copolymerization; Coplolymer; Polymerization; α-methylstyrene; Catalyst system