Original Article
, Volume: 16( 1)Coordination of Tellurium(IV) with Schiff Base Derived from o-Vanillin and 3-Aminopyridine
- *Correspondence:
- Garg S, Department of Chemistry, Maharshi Dayanand University, Rohtak, Haryana, India, Tel: 9896091443; E-mail: sapanagarg1511@gmail.com
Received: November 03, 2017; Accepted: February 24, 2018; Published: February 27, 2018
Citation: Malik A, Goyat G, Vikas K, Verma KK and Garg S. Coordination of Tellurium(IV) with Schiff Base Derived from o-Vanillin and 3-Aminopyridine. Int J Chem Sci. 2018;16(1):249
Abstract
Seven new organyltellurium(IV) complexes with monobasic ON bidentate Schiff base ligand have been synthesized. The Schiff base ligand (3-APY-{o-VanH}) was prepared by condensation of o-vanillin with 3-aminopyridine. The newly synthesized organyltellurium(IV) complexes and Schiff base have been characterized by elemental analyses, conductivity measurements, FT-IR and 1H NMR spectral studies. The structure of the complexes obtained was confirmed by FT-IR and proton NMR which exhibited pentacoordinated tellurium centre having Ψ-trigonal bipyramidal geometry. Schiff base as well as their organyltellurium(IV) complexes were also evaluated for their antimicrobial activities in vitro against Gram-positive bacteria (Staphylococcus aureus and Streptococcus pyogenes), Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli) and fungii Candida albicans, Aspergillus niger and Aspergillus
Keywords
o-Vanillin, Schiff base ligand, Organyltellurium(IV) complexes, Monobasic, Biological activities
Introduction
Schiff base ligands are well-known to be good chelating agents as bidentate, tridentate or polydentate ligands, particularly when the group such as –OH/-SH are present close to azomethine group, resulting in the formation of five or six membered ring complexes [1-6]. Schiff bases are reported to have biological activities like antibacterial [7-15], antifungal [7,9-12,16], antitumor [10,17,18], antiviral [19-21], anti-HIV [22], herbicidal [23] and anti influenza A virus [24] activities.
Tellurium(IV) chloride is also known to form adducts with amides [25,26] and thiourea [27], thus reflecting its acceptor behaviour [1,2,28-33]. Also, organyltellurium(IV) chlorides are known [1,2,28-50] to behave as lewis acids and form complexes with several N?, O? and S? donor bases. In view of this, we have investigated the reactions of tellurium(IV) chloride and organyltellurium(IV) chlorides with o-vanillin-3-aminopyridine Schiff base (3-APY-{o-VanH}), to synthesize some new complexes of tellurium(IV).
Materials and Methods
All chemicals used were of Analytical Reagent grade. All preparations were carried out under an atmosphere of dry N2 atmosphere. The solvents were purified by standard method [51,52] before use. The purity of compounds was checked by TLC using Silica gel-G (Merck). Melting points were determined in open capillary tube and are uncorrected.
4-Methoxyphenyltellurium(IV) trichloride [53,54], bis(p-methoxyphenyl)tellurium(IV) dichloride [54,55], 4-hydroxyphenyltellurium(IV) trichloride [56], bis(p-hydroxyphenyl)tellurium(IV) dichloride [56], 3-methyl-4-hydroxyphenyltellurium(IV) trichloride [57] and bis(3-methyl-4-hydroxyphenyl)tellurium(IV) dichloride [57] were prepared by the reactions of tellurium tetrachloride (Aldrich) with corresponding arenes i.e., anisole, phenol, o-cresol respectively, by the methods reported in the literature [53-57].
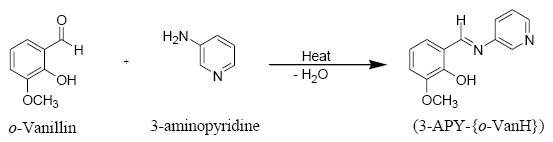
Preparation of o-vanillin-3-aminopyridine Schiff base (3-APY-{o-VanH})
The Schiff base was prepared by mixing equimolecular quantity of o-vanillin (0.08 mole, 12.17 g) and 3-aminopyridine (0.08 mole, 7.52 g) in 25 ml methanol in a round bottomed flask equipped with a condenser [58]. The reaction mixture was refluxed on wate rbath for 4 hours. After completion of reaction, the reaction mixture was cooled, filtered and dried in a desiccator over anhydrous CaCl2 and recrystallized from methanol, yellowish crystalline product was obtained.
Preparation of complexes
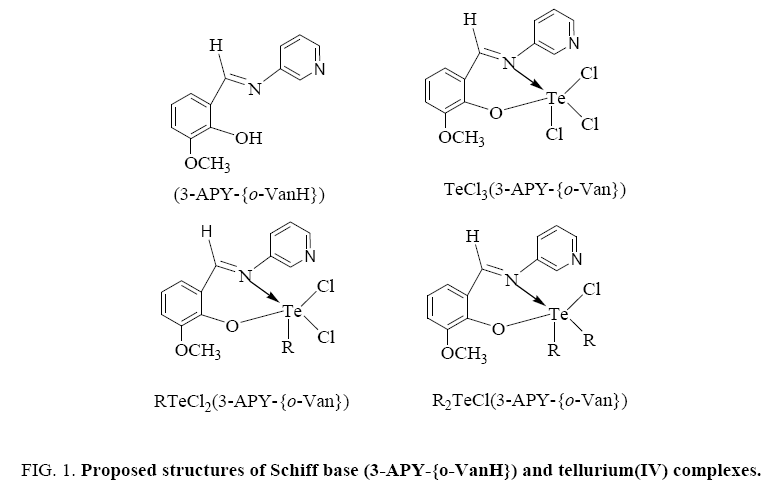
Tellurium tetrachloride, organyltellurium(IV) trichlorides and diorganyltellurium(IV) dichlorides, when reacted with Schiff base (3-APY-{o-VanH}) form solid complexes as described below:
[TeCl3(3-APY-{o-Van})], [RTeCl2(3-APY-{o-Van})] and [R2TeCl(3-APY-{o-Van})]
The solid complexes were prepared by addition of 5 mmol tellurium(IV) derivatives in about 25 mL anhydrous methanol to a hot solution of 5 mmol Schiff base (3-APY-{o-VanH}) in about 25 mL methanol with continuous stirring. The reaction mixture was refluxed on steam bath for 4 hours. The excess solvent was distilled off to obtain the desired products which were recrystallized from dry methanol. The coloured complexes crystallized out, which were filtered, washed with dry methanol and dried in a vacuum desiccator over P4O10.
Physical studies
Carbon, hydrogen and nitrogen analyses were obtained microanalytically from SAIF, Panjab University Chandigarh on a Thermo Finnigan CHNS analyser. Conductance studies were performed under dry condition in DMSO at 25 ± 2°C with a dip type conductivity cell on a microprocessor-based conductivity bridge type MICROSIL. Infrared spectra (4000-40 cm-1) were recorded in KBr and Polyethylene pellets for Mid-IR and Far-IR respectively, on a F.T. Infra-Red Spectrometer Model Nicolet IS50 (Thermo Scientific). Proton NMR Spectra were recorded in DMSO-d6 using tetramethylsilane as an internal reference on BRUKER AVANCE II 400 NMR spectrometer from CIL, Guru Jambeshwar University of Science and Technology, Hissar, Haryana, India.
Results and Discussion
TeCl4 when heated with anisole [53-55], phenol [56] and o-cresol [57,58] (R?H) appears to undergo the Friedel Craft type condensation reaction where by TeCl3+ unit attacks a position para to the methoxy/hydroxyl group in the aromatic ring, thus resulting in the formation of organyltellurium(IV) trichlorides and diorganyltellurium(IV) dichlorides.
Preparation of Schiff Base (3-APY-{o-VanH}), by the reaction of o-vanillin with 3-aminopyridine can be represented by following equation.
Schiff Base reacts with tellurium(IV) chloride, organyltellurium(IV) trichlorides and diorganyltellurium(IV) dichlorides in 1:1 molar ratio to yield the corresponding organyltellurium(IV) complexes.
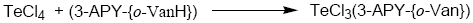
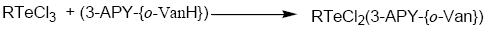
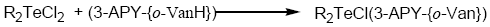
All the tellurium (IV) complexes are colored, crystalline solids, stable at room temperature and non-hygroscopic in nature. They are insoluble in non-polar and less polar organic solvents, but are soluble in polar donor solvents like DMF, DMSO etc. The analytical data along with their physical properties are presented in Table 1.
| Compoundno. | Complex (R) |
Empirical formula (Formula Wt.) |
Colour yield, (%) |
M. Pt. (°C)dec. |
Analyses % found (Calculated) | ΛM at ca. 10-3M S cm2mol-1 in DMSO |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Te | Cl | ||||||
| Schiff Base | (3-APY-{o-VanH}) | C13H12N2O2 (228.15) |
Yellowish (90) |
118-120 | 68.24 (68.43) |
5.32 (5.26) |
12.17 (12.28) |
- | - | - |
| I | TeCl3(3-APY-{o-Van}) | C13H11Cl3N2O2Te (461.24) |
Dark Brown (85) |
204-206 | 33.71 (33.85) |
2.45 (2.38) |
6.15 (6.07) |
27.53 (27.66) |
22.95 (23.09) |
21.16 |
| II | RTeCl2(3-APY-{o-Van}) (4-methoxyphenyl) |
C20H18Cl2N2O3Te (532.81) |
Reddish brown (76) |
138-140 | 44.92 (45.08) |
3.22 (3.38) |
5.18 (5.26) |
24.05 (23.95) |
13.03 (13.14) |
18.09 |
| III | RTeCl2(3-APY-{o-Van}) (4-hydroxyphenyl) |
C19H16Cl2N2O3Te (518.80) |
Brick Red (69) |
153-155 | 43.77 (43.98) |
3.23 (3.08) |
5.27 (5.40) |
24.67 (24.59) |
13.54 (13.69) |
12.01 |
| IV | RTeCl2(3-APY-{o-Van}) (3-methyl-4-hydroxyphenyl) |
C20H18Cl2N2O3Te (532.81) |
Brown (74) |
190-192 | 44.89 (45.08) |
3.45 (3.38) |
5.34 (5.26) |
23.86 (23.95) |
13.20 (13.14) |
19.12 |
| V | R2TeCl(3-APY-{o-Van}) (4-methoxyphenyl) |
C27H25ClN2O4Te (604.38) |
Dark Yellow (79) |
184-186 | 53.52 (53.65) |
4.27 (4.14) |
4.53 (4.64) |
21.23 (21.11) |
5.82 (5.87) |
32.19 |
| VI | R2TeCl(3-APY-{o-Van}) (4-hydroxyphenyl) |
C25H21ClN2O4Te (576.36) |
Brown (82) |
192-194 | 51.87 (52.09) |
3.51 (3.64) |
4.74 (4.86) |
22.05 (22.14) |
6.09 (6.16) |
42.90 |
| VII | R2TeCl(3-APY-{o-Van}) (3-methyl-4-hydroxyphenyl) |
C27H25ClN2O4Te (604.38) |
Reddish Brown (78) |
144-146 | 53.45 (53.65) |
4.03 (4.14) |
4.56 (4.64) |
21.02 (21.11) |
5.75 (5.87) |
44.36 |
Table 1: Analytical data, molar conductance and physical properties for Schiff base (3-APY-{o-VanH}) complexes of tellurium(IV). Values of ΛM reported [59,60] for 1:1 electrolytes in DMSO=50-70 S cm2 mol-1.
Conductance studies
The molar conductance (ΛM) data for organyltellurium(IV) Schiff base complexes in DMSO are compiled in Table 1. The ΛM values at ca. 10-3 M of complexes lies in the range 12.01-44.36 S cm2 mol-1 which predict the non-electrolyte to 1:1 weak electrolyte type behavior [59,60] of these Schiff base complexes in DMSO, probably due to ionization into TeCl2(3-APY-{o- Van})+/RTeCl(3-APY-{o-Van})+/R2Te(3-APY-{o-Van})+ and Cl - in DMSO. The higher ΛM values for some complexes may be due to steric factors and donor behavior of DMSO to result in probable dissociation into solvated cation and 3-APY-{o- Van}- along with Cl- in DMSO. This conductance behavior of tellurium(IV) Schiff base complexes is different from those of transition metal complexes [61] which are reported to be non-electrolytes.
Infrared spectra
The IR spectral data of Schiff base and its complexes with organyltellurium(IV) chlorides are recorded in solid state and selected bands of diagnostic importance are collected in Table 2. The band at 1616 cm-1 of the ligand is assigned [25,58,62] to the stretching vibration of the azomethine group. When the spectra of complexes are compared with those of uncomplexed Schiff base ligand the υ(C=N) band is shifted to lower frequency [16,58,62-64] this indicates that imine nitrogen [65,66] is coordinated to the metal centre. The band around 3085 cm-1, in free ligand ascribed to the υ(OH) of phenolic group disappear on complexation with tellurium atom and shows that phenolic group of o-vanillin is involved in bonding after deprotonation. The two new bands appear in range 289-294 cm-1 and 408-419 cm-1 assigned to υ(Te-O) [63,64,67] and υ(Te-N) mode of vibration. Thus, IR data predict to monobasic bidentate nature of the Schiff base (3-APY-{o-VanH}) involving azomethine nitrogen atom and phenolic oxygen after deprotonation giving rise to six membered chelate ring with the penta coordinated tellurium centre.
| Compound No. |
(Phenolic) ν (OH) | (Azomethine) ν(C=N) | ν(Te=O) | ν(Te=N) |
|---|---|---|---|---|
| (3-APY- {o-VanH}) |
3085 s | 1616 s | - | - |
| I | - | 1606 s | 289m | 419m |
| II | - | 1609 s | 290 s | 419 s |
| III | - | 1609m | 289m | 418 s |
| IV | - | 1608sh | 290 s | 419m |
| V | - | 1606 s | 294 s | 408mb |
| VI | - | 1609 s | 291m | 419 m |
| VII | - | 1609 s | 292 s | 414s |
s: Strong; m: Medium; b: Broad;sh: Shoulder
Table 2: Important IR data (cm-1) of the Schiff base (3-APY-{o-VanH}) and Complexes.
1H NMR spectra
The 1H NMR spectral data of ligand (3-APY-{o-VanH}) and complexes were recorded in DMSO-d6 and are given in Table 3. The proton peak of phenolic –OH group at 13.124 δ ppm had disappeared, which suggest that the hydroxyl group coordinates to the metal centre after deprotonation. The singlet at 8.660 δ ppm (s, 1H) attributed to the imine hydrogen in the ligand shift to downfield side in complexes clearly demonstrate [42,43,62] the coordination of azomethine nitrogen to tellurium. Independent assignments to the aryl protons of (3-APY-{o-VanH}) and RTe/R2Te are not possible due to overlapping of signals in this region.
| Compound number |
(Phenolic) -OH δ ppm |
(Azomethine) -HC=N δ ppm |
(Ar rings protons) δ ppm |
-CH3/-OCH3* δ ppm |
-OH of RTe/R2Te δ ppm |
|---|---|---|---|---|---|
| (3-APY- {o-VanH}) |
13.124 (s, 1H) | 8.660 (s, 1H) | 6.899-8.582 (cm, 7H) | 3.954 (s,3H*) | - |
| I | - | 10.140 (s, 1H) | 6.841-7.944 (cm, 7H) | 3.749 (s,3H*) | - |
| II | - | 10.140 (s, 1H) | 6.832-8.230 (cm, 11H) | 3.414 (s,6H*) | - |
| III | - | 10.138 (s, 1H) | 6.838-8.229 (cm, 11H) | 3.424 (s,3H*) | 9.089 (s,1H) |
| IV | - | 10.273 (s, 1H) | 6.851-8.093 (cm, 10H) | 2.513 (s,3H)/3.848 (s,3H*) | 9.102 (s,1H) |
| V | - | 10.147 (s, 1H) | 6.811-7.929 (cm, 15H) | 3.402 (s,9H*) | - |
| VII | - | 10.145 (s, 1H) | 6.850-8.095 (cm, 13H) | 2.510 (s,6H)/3.437 (s,3H*) | 9.098 (s,2H) |
s: Singlet; cm: Complex Multiplet; Spectra of compound number VI not well resolved due to poor solubility
Table 3: 1H NMR spectral data of Schiff base (3-APY-{o-VanH}) and complexes in DMSO-d6. s=singlet, cm=complex multiplet. Spectra of compound number VI not well resolved due to poor solubility.
Conclusion
The Schiff base (3-APY-{o-VanH}) and newly synthesized organyltellurium(IV) Schiff base complexes were screened in vitro antimicrobial potential against Gram +ve bacteria (S. aureus MTCC 96 and S. pyogenes MTCC 442), Gram -ve bacteria (P. aeruginosa MTCC 1688 and E. coli MTCC 443) strain; fungal strains C. albicans MTCC 227, A. niger MTCC 282 and A. clavatus MTCC 1323 by “Broth Dilution Method”. The results were recorded in terms of MIC values are present in Table 4. Comparative study of MIC value for Schiff base (3-APY-{o-VanH}) and their tellurium(IV) complexes indicates that some complexes exhibit higher antibacterial activity than Schiff base itself. It has been observed that the complex no. VI [R2TeCl(3-APY-{o-Van})]: where R=4-hydroxyphenyl,) shows stronger antifungal activity than Schiff base itself against A. niger and A. Clavatus.
| Compound number | Bacterial strain | Fungal strain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus MTCC 96 |
S. pyogenes MTCC 442 |
P. aeruginosa MTCC 1688 |
E. coli MTCC 443 |
C. albicans MTCC 227 |
A. niger MTCC 282 |
A. clavatus MTCC 1323 |
||||
| (3-APY- {o-VanH}) |
250 | 250 | 200 | 200 | 250 | >1000 | >1000 | |||
| I | 250 | 250 | 125 | 100 | >1000 | >1000 | >1000 | |||
| II | 250 | 200 | 100 | 125 | 500 | 1000 | 1000 | |||
| III | 200 | 200 | 250 | 250 | 500 | 1000 | 1000 | |||
| VI | 500 | 500 | 250 | 250 | 1000 | 250 | 500 | |||
| Standard drugs | ||||||||||
| Ampicillin | 250 | 100 | 100 | 100 | - | - | - | |||
| Chloramphenicol | 50 | 50 | 50 | 50 | - | - | - | |||
| Nystatin | - | - | - | - | 100 | 100 | 100 | |||
| Greseofulvin | - | - | - | - | 500 | 100 | 100 | |||
Table 4: Minimum inhibitory concentration MIC (μg/mL) of Schiff base (3-APY-{o-VanH}) and complexes.
On the basis of these studies, the proposed structures for the complexes are as below (Figure 1).
Acknowledgement
The authors are grateful to M. D. University, Rohtak for providing the necessary facilities. One of the authors (AM) is also thankful to HSCST, Haryana for providing a fellowship. We also thank Microcare Laboratory, Surat, for providing antimicrobial activities.
References
- Goyat G, Malik A, Garg S, et al. Synthesis, characterization and antimicrobial studies on some complexes of tellurium (IV) with Schiff base derived from isatin and 2-aminophenol. Int J Chem Sci. 2016;14(3):1498-510.
- Goyat G, Malik A, Garg S, et al.Tellurium (IV) complexes of tridentate(ONS) Schiff base derived from isatin and 2-aminothiophenol. J Chem Pharm Res. 2016;8(4):218-23.
- Canpolat E, Kaya M. Studies on mononuclear chelates derived from substituted Schiff base ligand: Synthesis and characterization of a new 5-methoxysalicylidene-p-aminoacetophenoneoxime and its complexes with Co(II), Ni(II), Cu(II) and Zn(II). Russ J Coord Chem. 2005;31(11):790-4.
- Booysena IN, Maikoa S, Akermana MP, et al. Ruthenium(II/IV) complexes with potentially tridentatesSchiff base chelates containing the uracil moiety. J Coord Chem. 2013;66(20):3673-85.
- Barwiolek M, Szlyk E, Surdykowski A, et al. New nickel(II) and copper(II) complexes with unsymmetrical Schiff bases derived from (1R,2R)(-)cyclohexanediamine and the application of Cu(II) complexes for hybrid thin layers deposition. Dalton Trans. 2013;42:11476-87.
- Fernandez-G JM, Portilla FDR, Garcia BQ, et al. The structures of some ortho-hydroxy Schiff base ligands. J MolStruct. 2001;561(1):197-207.
- Sridhar SK, Saravanan M, Ramesh A. Synthesis and antibacterial screening of hydrazones,Schiff and Mannich bases of isatin derivatives.Eur J Med Chem. 2001;36:615-25.
- Mladenova R, Ignatova M, Manolova N, et al. Preparation, characterization and biological activity of Schiff base compounds derived from 8-hydroxyquinoline-2-carboxaldehyde. EurPolym J. 2002;38:989-99.
- Panneerselvem P, Nair RR, Vijayalakshmi G, et al. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur J Med Chem. 2005;40:225-9.
- Walsh OM, Meegan MJ, Prendergast RM, et al. Synthesis of 3-acetoxyazetidin-2-ones and 3-hydroxyazetidin-2-ones with antifungal and antibacterial activity. Eur J Med Chem. 1996;31:989-1000.
- Pandeya SN, Sriram D, Nath G, et al. Synthesis,antibacterial,antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4’-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur J Pharm. 1999;9:25-31.
- Pandeya SN, Sriram D, Nath G, et al. Synthesis,antibacterial,antifungal and anti-HIVactivities of Schiff and mannich bases of isatin derivatives with 3-amino-2-methylmercaptoquinazolin-4(3H)-one. Pharm ActaHelv. 1999;74:11-17.
- Panneerselvam P, Rather BA, Reddy DRS, et al. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6,8-dibromo-2-phenylquinazolin-4(3H)-ones. Eur J Med Chem. 2009;44(5):2328-33.
- Rai BK, Kumar A. Synthesis, characterization and biocidal activity of some Schiff base and its metal complexes of Co(II), Cu(II) and Ni(II). Orient J Chem. 2013;29(3):1187-91.
- Singh K, Barwa MS, Tyagi P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur J Med Chem. 2006;41(1):147-53.
- Ramesh R, Maheswaran S. Synthesis, spectra, dioxygenaffinity and antifungal activity of Ru(III) Schiff base complexes. J InorgBiochem. 2003;96:457-62.
- Liu MC, Lin TS, Sartorelli AC. Synthesis and antitumor activity of amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J Med Chem. 1992;35:3672-7.
- Hodnett EM, Dunn JW. Structure-antitumor activity correlation of some Schiff bases. J Med Chem. 1970;13:768-70.
- Kumar KS, Ganguly S, Veerasamy R, et al. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4(3)H-ones. Eur J Med Chem. 2010;45(11):5474-9.
- Jarrahpour A, Khalili D, DeClercq E, et al. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-schiff bases of isatin and their derivatives. Molecules. 2007;12(8):1720-30.
- Mishra PM. Synthesis, structural elucidation of complexes of some 3d-series divalent transition metals with 2-hydroxy-4-nitroacetophenone hydrazone, Schiff Base Ligand. Orient J Chem. 2013;29(2):677-83.
- Vicini P, Geronikaki A, Incerti M, et al. Synthesis, biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem. 2003;11:4785-9.
- Holla BS, Rao BS, Shridhara K, et al. Studies on arylfuran derivatives. Part XI. Synthesis, characterization and biological studies on some mannich bases carrying 2,4-dichlorophenylfurfural moiety. Farmaco. 2000;55(5):338-44.
- Zhao X, Li C, Zeng S, et al. Discovery of highly potent agents against influenza Virus. Eur J Med Chem. 2011;46(1):52-7.
- Malhotra KC, Paul KK.Ionic adducts of amides. Curr Sci. 1969;38:266.
- Peirier M.Vincontini, Anal Acad. Brazil Cinene. 1971;43:119.
- Aynsley EE, Campbell WA. Complexes of thiourea containing tellurium. J Chem Soc. 1958;pp:3290-3.
- Goyat G, Garg S, Verma KK. Complexes of tellurium (IV) with Isatin-Aniline Schiff base. ChemSci Trans. 2016;5(2):479-87.
- Goyat G, Garg S, Verma KK. Investigations on some isatin-p-toluidine Schiff base complexes of tellurium (IV). Res. J. Pharm. BiolChem Sci. 2016;7(2):869-77.
- Goyat G, Malik A, Garg S, et al. Studies on some tellurium (IV) complexes of N, N’-Bis(Indol-2-oxo-3-ylidine)-1,2-diaminoethane. Int J Chem Sci. 2016;14(1):387-98.
- Goyat G, Malik A, Garg S, et al. Studies on some propylenediamine-bis(isatin) Schiff base complexes of tellurium (IV). Der PharmaChemica. 2016;8(2):198-203.
- Malik A, Verma KK, Garg S. Studies on some tellurium (IV)complexes of salicylaldehyde-2-aminopyridine Schiff base.Res J Pharm BiolChem Sci. 2017;8(6)190-8.
- Malik A, Verma KK, Garg S. Coordination of tellurium (IV) with Schiff Base derived from o-Vanillin and 3-aminopyridine. ChemSci Trans. 2015.
- Wynne KJ, Pearson PS. Complexes of organotelluriumtrihalides with tetramethylthiourea. Inorg Chem. 1971;10:2735-39.
- Wynne KJ, Pearson PS. Preparation of methyltrihalogeno(tetramethylthiourea) tellurium (IV) compounds: Pentaco-ordinate tellurium. J ChemSocCommun. 1970;pp:556-7.
- Wynne KJ, Clark AJ, Berg M. Chalcogen chemistry. Part VIII. Complexes of arylselenium and aryltelluriumtrichlorides with pyridine, 4-picoline and 4-picoline-N-oxide. J ChemSoc Dalton. 1972;pp:2370-8.
- Clark ER, Collet AJ, Naik DG, Tetraethyldithio-oxamide complexes of tellurium (IV), J. Chem. Soc. Dalton, 1973; 1961.
- Srivastava TN, Singh M, Singh HB. Complexes of organotellurium chlorides with N, P, O and S-donors. Indian J Chem. 1982;21A:307.
- Srivastava TN, Srivastava RC, Srivastava M. Complexes of organotellurium chlorides with N, O, and S-donors. Indian J Chem. 1982;21A:539.
- Srivastava TN, Srivastava RC, Srivastava VK. Studies on molecular adducts of hydroxyphenyltellurium (IV)trichlorides. J Indian Chem Soc. 1983;60:891.
- Garad MV. Molecular complexes of aryltellurium (IV) chlorides. Polyhedron. 1985;4:1353.
- Verma KK, Reena A.Complexes of 4-hydroxyphenyltellurium trihalides with piperidine, dimethylformamide and thiourea.Synth React Inorg Met Org Chem. 1999;29:499-512.
- Verma KK, Dahiya R, Soni D. Synthesis and characterization of complexes of some hydroxyaryltelluriumtrichlorides with N-donor ligands. Synth React Inorg MetOrg Chem. 1999;29:1033-52.
- Verma KK, Dahiya R. Synthesis and characterization of pyridine and bipyridyl complexes of 4-hydroxyphenyltellurium trihalides. Synth React Inorg Met Org Chem. 1999;29:1299-314.
- Verma KK, Reena A. Studies on thiourea complexes of some hydroxyaryltelluriumtrichlorides. Phosphorus, Sulfur and Silicon and the Related Elements. 1999;148:227-34.
- Verma KK. Study on picoline complexes of p-Hydroxyphenyltellurium (IV)trihalides. Int J Chem Sci. 2008;6:371-80.
- Srivastava S, Soni DK, Gupta HS. Some molecular adducts of dibenzyltellurium (IV) derivatives with nitrogen donor molecules. J Indian Chem Soc. 1996;73:255.
- Narwal JK, Chhabra S, Malik RK, et al. Studies on the pyrazine complexes of some diaryltelluriumdihalides. Oriental J Chem. 2013;29:1339-49.
- Chhabra S, Verma KK. Studies on 1,10-phenanthroline complexes of some diaryltelluriumdihalides. J Chem Pharm. Res. 2010;2:569-75.
- Vogel AI. A Text Book of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis. 3rdedn. Longmans, London. 1975.
- Morgan GT, Kellet RE. Interactions of tellurium tetrachloride and aryl alkyl ethers. Part II J Chem Soc. 1926;1080-8.
- Petragnani N, Stefani HA. Tellurium in Organic Chemistry. 2ndedn. Academic Press, London. 2007;pp:76.
- Bergman J. Tellurium in organic chemistry-A novel synthesis of biaryl. Tetrahedron. 1972;28:3323-1.
- Khandelwal BL, Kumar K, Berry FJ.Hydroxyphenyltellurium (IV) Halides.InorgChimActa. 1981;99:135-7.
- Khandelwal BL, Kumar K, Raina K. Synthesis and characterization of (methylhydroxyphenyl) tellurium (IV) halides. Synth React Inorg Met Org Chem. 1981;11:65-78.
- Abdel-Latif SA, Hassib HB, Issa YM. Studies on some salicylaldehyde Schiff base derivatives and their complexes with Cr(III), Mn(II), Fe(III), Ni(II) and Cu(II). SpectrochimActa(A). 2007;67:950-7.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. CoordChem Rev. 1971;7:81-122.
- Greenwood NN, Straughan BP, Wilson AE.Behaviour of tellurium (IV) chloride, bromide and iodide in organic solvents and structures of the species. J Chem Soc. 1968;2209.
- Srivastava KP, Singh A, Singh SK. Green and efficient synthesis, characterization and anti-bacterial activity of copper(II) complexes with unsymmetrical bidentate Schiff base ligands. J App Chem. 2014;7(4):16-23.
- Dharmaraj N. Ruthenium(II) complexes containing bidentateschiff bases and their antifungal activity. Trans Metal Chem. 2001;26:105-109.
- Verma KK, Soni D.Diaryltellurium (IV) carboxylates:Synthesis viatelluroxides and their characterization. Phosphorus, Sulfur and Silicon. 2000;166(1):231-41.
- Pant BC, McWhinnie WR, Dance NS. Organotellurium carboxylates and related compounds: Structural and synthetic considerations. J Organmetal Chem. 1973;63:305-10.
- Srivastava TN, Singh JD.Diorganotellurium (IV)bis(dimethylglyoximates). Indian J Chem. 1987;26A:260.
- Chauhan S, Garg S, Verma KK. Studies on some salicylhydroxamate complexes of aryltellurium (IV). ChemSci Trans 2016;5(2):431-41.
- Kulkarni YD, Srivastava S, Abdi SHR, et al. Synthesis and reactivity of some a,a-Bis(2-and-4-substituted benzoyl)tellurium dichlorides. Synth React Inorg Met Org Chem. 1985;15(8):1043-59.
- Khera B, Sharma AK, Kaushik NK.Bis(indenyl) titanium(IV) and zirconium(IV) complexes of monofunctionalbidentatesalicylidimines. Polyhedron. 1983;2(11):1177-80.
- Joshi KR, Rojivadiya AJ, Pandya JH. Synthesis and spectroscopic and antimicrobial studies of schiff base metal complexes derived from 2-hydroxy-3-methoxy-5-nitrobenzaldehyde. Int J Inorg Chem. 2014;10:1155-62.