Original Article
, Volume: 9( 6)Comparison of Methods for Calculating the Combustion Heat of Biopolymers and Vegetable Biomass
Michael Ioelovich*
Designer Energy Ltd, 2 Bergman Str, Rehovot, Israel
- *Corresponding Author:
- Michael Ioelovich, Designer Energy Ltd, 2 Bergman Str, Rehovot, Israel, Tel: 972-8936661; Fax: 972-89366614; E-mail: bd895892@zahav.net.il
Received Date: November 10, 2016; Accepted Date: November 21, 2016; Published Date: November 24, 2016
Citation: Ioelovich M. Comparison of methods for calculating the combustion heat of biopolymers and vegetable biomass. 2016;9(6): 107.
Abstract
In this paper various methods for calculation of gross and net heat of combustion for different biopolymers (lignin, cellulose, hemicelluloses, starch, pectin, proteins, lipids etc.) have been analyzed. The results showed that the calculation with use the energy released by combustion per gram of diatomic oxygen (Eq-parameter) is less accurate, because it gives a deviation from experimental values of more than 6%. In the case of calculations based on contribution of structural groups of polymers, the deviation for some samples may reach 6%. The lowest deviation in the range ±1% was obtained using an improved method of calculation, which is based on elemental composition of the polymers. Calculation of gross and net heat of combustion for biomass samples by the improved method was very close to experimental calorific values. It was found that combustion of plant biomass waste supplemented with waste plastic is preferable, since such combustion technology provides more thermal energy than single firing of biomass and is accompanied by less emission of carbon dioxide in comparison with separate burning of plastic waste only.
Keywords
Biopolymers; Biomass; Plastics; Chemical structure; Composition; Heat of combustion; Calorimetry; Calculation
Introduction
Mankind produces a huge amount of solid waste including several billion tons of plant-based waste (paper, cardboard, textile, sawdust, shavings, branches, straw, husk, etc.) and hundreds million tons of waste plastics annually [1]. Up to 50% of the waste is recycled, and about 20% is burned to generate the thermal energy [2].
In order to determine the true value of gross energy or enthalpy of combustion of organic materials, a special apparatus is required such as precise, but complex and expensive calorimeter. Moreover, the calorimetric experiments are long and require the introduction of various corrections and laborious calculations. Therefore to characterize fuel properties of organic substances, e.g. combustible waste, the approximate calculations of calorific value are performed using equations of Mendeleev or other researchers [3,4]. Estimating calculation method of combustion heat based on contribution of atoms and structural groups of polymers to thermal energy is also proposed [5]. Study of 49 synthetic polymers showed that the net heat (Eq, kJ) released by combustion per gram of diatomic oxygen has approximately constant value: Eq=13.10 ± 0.78 kJ per 1g of O2 [6,7]. Then, net heat of combustion (q, kJ/g) of the polymer sample can be calculated by the equation:
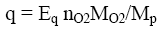 (1)
(1)
where MO2 = 32 is molecular weight of O2 and nO2 is number of O2 molecules consumed for complete combustion of one mole of the repeat unit of polymer having molecular weight Mp.
As known, vegetable biomass is a complex of natural biopolymers such as cellulose, hemicelluloses, lignin, starch, pectin, proteins, lipids and some others. Therefore, to estimate fuel properties of the biomass waste it is advisable to know beforehand the calorific value of the individual biopolymers. For this purpose it is necessary to perform a comparative analysis of various methods for calculating the combustion heat value of different biopolymers.
Experimental Materials
Various samples of biopolymers and plant biomass were studied (TableS 1 and 2).
| Polymer | Abbreviation | Repeat unit | Mp |
|---|---|---|---|
| Gluten of wheat | GLT | C5H6O2N | 112 |
| Xylan of birch | XYL | C5H8O4 | 132 |
| Mannan of spruce | MAN | C6H10O5 | 162 |
| Cellulose of cotton | CEL | C6H10O5 | 162 |
| Starch of potatoes | STR | C6H10O5 | 162 |
| Pectin of citrus | PEC | C6H8O6 | 176 |
| Lignin Bjorkman of spruce | LIG | C10H11O3 | 179 |
| Cutin of aspen bark | CUT | C54H104O6 | 848 |
| Suberin of aspen bark | SUB | C72H140O6 | 1100 |
Table 1: List of used biopolymers.
| Biomass | Cellulose | Hemi | Lignin | Extractives | Minerals |
|---|---|---|---|---|---|
| Softwood | 0.48 | 0.20 | 0.28 | 0.02 | 0.02 |
| Hardwood | 0.45 | 0.25 | 0.23 | 0.03 | 0.04 |
| Bagasse | 0.40 | 0.31 | 0.20 | 0.03 | 0.06 |
| Corn stover | 0.38 | 0.33 | 0.2 | 0.02 | 0.07 |
| Cardboard | 0.60 | 0.12 | 0.18 | 0.03 | 0.07 |
| Wrapping paper | 0.73 | 0.07 | 0.05 | 0.03 | 0.12 |
Table 2: Chemical composition of biomass (wt. p.).
Methods
Gross combustion heat (Q) of the samples was determined using a stainless steel calorimetric bomb having volume 0.320 dm3 at oxygen pressure of 3.05 MPa with 1.00 cm3 of deionized water added to the bomb [8]. The combustion measurements were carried out by an isothermal water calorimeter at 298.15 K with accuracy ±0.001 K. The value of the energy equivalent of the calorimeter determined by standard benzoic acid was 15802.3 ± 0.9 J/K. The true mass of the sample used in each experiment was determined from the mass of the producedCO2. The system was thermo stated for 1 h. After ignition, the temperature rise was measured within 1 h. All needed corrections were introduced. Conventional procedure was used to adjust the experimental combustion energy to standard conditions: T=298.15 K and p=0.1 MPa. For each sample five experiments were performed to calculate an average value of combustion enthalpy and standard deviation.
The net combustion heat (q) of the samples was calculated by the equation [7]:
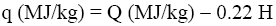 (2)
(2)
Where H is percentage of hydrogen in the sample.
The experimental results were compared with Q and q values obtained by various calculation methods based on equations of Mendeleev (Eq. 3 and 4) [3], Parikh et al. (Eq. 5 and 6) [4], improved Eq. 7 and 8, on Eq-parameter (Eq. 1 and 9) [5-7], as well as on structural groups of polymers [5].
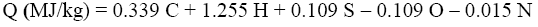 (3)
(3)
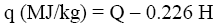 (4)
(4)
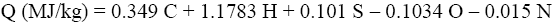 (5)
(5)
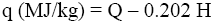 (6)
(6)
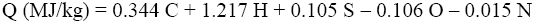 (7)
(7)
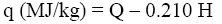 (8)
(8)
Where C, H, O, S, N is percentage of atoms in dry organic material.
Gross heat value can be also calculated using the Eq-parameter:
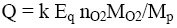 (9)
(9)
Where k ≈0.075 is coefficient of transition from net to gross heat of combustion.
To implement the method of structural groups, the contributions of these groups to the heat of combustion should be used (Table 3).
| Group | Qi, kJ/mol group | Group | Qi, kJ/mol group |
|---|---|---|---|
| -CH3 | 775 | -COO- | 112 |
| -CH2- | 670 | -C=O | 259 |
| -CH- | 518 | -NH- | 77 |
| -C- | 431 | -O- | -132 |
| -H | 190 | -OH | -108 |
Table 3: Structural groups and their contributions to gross heat of combustion [5].
Gross heat of combustion was calculated by the equation:
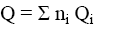 (10)
(10)
Then, the net combustion heat of the samples was calculated:
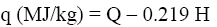 (11)
(11)
Results and Discussion
Results of experimental determination of gross and net heat of combustion for investigated biopolymers are shown in Table 4.
| Polymer | Formula | Mp | n(O2) | Q, MJ/kg | q, MJ/kg |
|---|---|---|---|---|---|
| PEC | C6H8O6 | 176 | 5 | 13.73 | 12.76 |
| XYL | C5H8O4 | 132 | 5 | 17.80 | 16.38 |
| STR | C6H10O5 | 162 | 6 | 17.53 | 16.23 |
| MAN | C6H10O5 | 162 | 6 | 17.50 | 16.20 |
| CEL | C6H10O5 | 162 | 6 | 17.45 | 16.12 |
| GLT | C5H6O2N | 112 | 5.5 | 21.87 | 20.64 |
| LIG | C10H11O3 | 179 | 11.25 | 27.94 | 26.45 |
| CUT | C54H104O6 | 848 | 77 | 40.51 | 37.34 |
| SUB | C72H140O6 | 1100 | 104 | 42.56 | 39.25 |
Table 4: Calorimetric data for gross (Q) and net (q) combustion heat of biopolymers.
The experimental values of the combustion heat (EXP) were compared with the results of calculations using various methods (TableS 5 and 6). Analysis of the results showed that the calculations by Eq. (1) and Eq. (9) with use the Eq-parameter are less accurate because they give a deviation from experimental values of more than 6%. In the case of calculations by eq. (10) and Eq. (11) based on contribution of structural groups of polymers, the deviation for some samples may reach 6%. The lowest deviation in the range ±1% was obtained using an improved method of calculation by Eq. (7) and (8), which is based on elemental composition of the polymers.
| Polymer | EXP | Eq. (3) | Eq. (5) | Eq. (7) | Eq. (9) | Eq. (10) |
|---|---|---|---|---|---|---|
| PEC | 13.73 | 13.61 | 13.97 | 13.78 | 12.80 | 13.70 |
| XYL | 17.80 | 17.74 | 18.00 | 17.87 | 17.07 | 17.14 |
| STR | 17.53 | 17.43 | 17.67 | 17.55 | 16.69 | 16.49 |
| MAN | 17.50 | 17.43 | 17.67 | 17.55 | 16.69 | 16.49 |
| CEL | 17.45 | 17.43 | 17.67 | 17.55 | 16.69 | 16.49 |
| GLT | 21.87 | 21.72 | 21.98 | 21.80 | 22.12 | 21.90 |
| LIG | 27.94 | 27.66 | 28.10 | 27.75 | 28.32 | 27.71 |
| CUT | 40.51 | 40.05 | 40.66 | 40.11 | 40.91 | 40.94 |
| SUB | 42.56 | 42.18 | 42.83 | 42.62 | 42.60 | 42.52 |
Table 5: Comparison of experimental and calculated values of gross combustion heat of biopolymers (Q, MJ/kg).
| Polymer | EXP | Eq. (4) | Eq. (6) | Eq. (8) | Eq. (1) | Eq. (11) |
|---|---|---|---|---|---|---|
| PEC | 12.76 | 12.62 | 12.97 | 12.80 | 11.91 | 12.71 |
| XYL | 16.38 | 16.40 | 16.65 | 16.53 | 15.88 | 15.80 |
| STR | 16.23 | 16.07 | 16.31 | 16.18 | 15.52 | 15.18 |
| MAN | 16.20 | 16.07 | 16.31 | 16.18 | 15.52 | 15.18 |
| CEL | 16.12 | 16.07 | 16.31 | 16.18 | 15.52 | 15.18 |
| GLT | 20.64 | 20.42 | 20.83 | 20.61 | 24.33 | 20.71 |
| LIG | 26.45 | 26.42 | 26.78 | 26.60 | 26.35 | 26.34 |
| CUT | 37.34 | 37.21 | 37.63 | 37.42 | 38.06 | 38.24 |
| SUB | 39.25 | 38.65 | 39.00 | 38.83 | 39.63 | 39.72 |
Table 6: Comparison of experimental and calculated values of net combustion heat of biopolymers (q, MJ/kg).
Calculation of gross and net heat of combustion for biomass samples was performed taking into account chemical composition of the samples (Table 2):
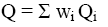 (12)
(12)
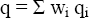 (13)
(13)
Where wi is weight part of individual components of the biomass; Qi and qi are gross and net combustion heat calculated by the improved method (Eq. 7 and 8).
As can be seen, the calculated heat values for various biomass samples were very close to experimental values (Table 7).
| Biomass | Q, MJ/kg | q, MJ/kg | ||
|---|---|---|---|---|
| EXP | CALC | EXP | CALC | |
| Softwood | 20.41 | 20.53 | 19.11 | 19.18 |
| Hardwood | 19.76 | 19.90 | 18.53 | 18.61 |
| Bagasse | 19.35 | 19.23 | 18.02 | 17.97 |
| Corn stover | 18.77 | 18.82 | 17.44 | 17.54 |
| Cardboard | 18.48 | 18.45 | 17.21 | 17.24 |
| Wrapping paper | 16.69 | 16.65 | 15.56 | 15.45 |
Table 7: Comparison of experimental (EXP) and calculated (CALC) values of combustion heat of biomass samples.
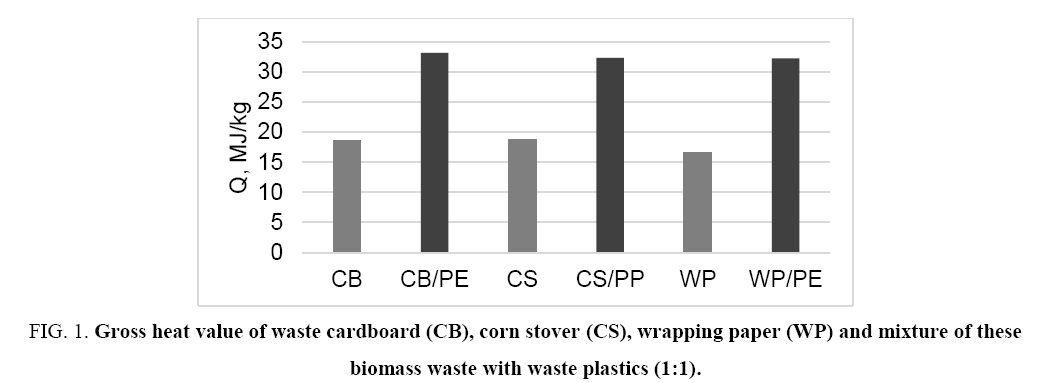
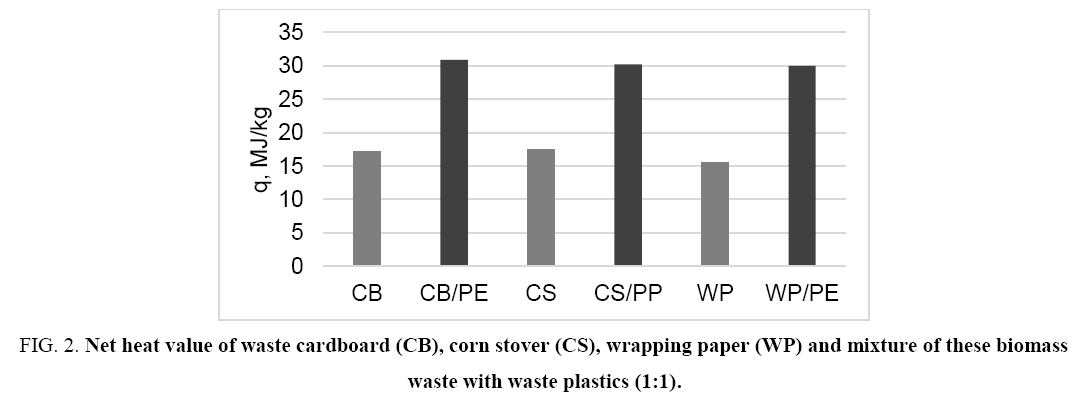
The main problem for using the biomass waste as solid fuel is relatively low heat output. One of the ways to solve this problem is to obtain a mixed solid fuel containing along with biomass waste also an additive of widespread waste plastics such as PE and/or PP. On the one hand it allows releasing the environment from solid waste, and on the other hand the use of mixed fuel provides a greater amount of thermal energy ( Figure 1 and 2).
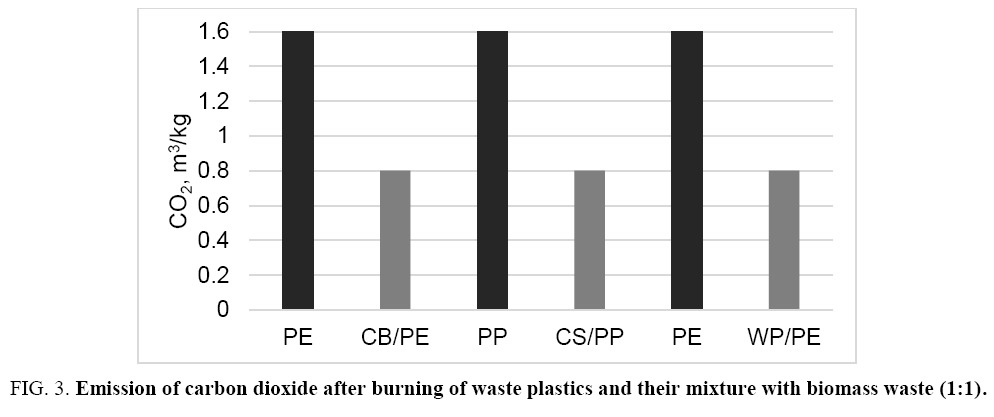
Furthermore, since the biomass is neutral for emission of carbon dioxide into the environment, burning of the mixed fuel results in reduction of carbon dioxide emission by half in comparison with a separate burning of plastic waste only ( Figure 3).
Conclusion
Gross and ten heat of combustion for different biopolymers such as lignin, cellulose, hemicelluloses, starch, pectin, proteins, lipids, as well as some biomass have been determined.
The experimental values of the combustion heat were compared with the results of calculations using various methods. The results showed that the calculation with use the energy released by combustion per gram of diatomic oxygen (Eq-parameter) is less accurate, because this method gives a deviation from experimental values of more than 6%. In the case of calculation based on contribution of structural groups of polymers, the deviation may reach 6%. The lowest deviation in the range ±1% was obtained using an improved calculation method of gross (Q) and net (q) heat values, which is based on elemental composition of the polymers (Eq. 7 and 8).
Values of combustion heat for biomass samples calculated by the improved method were very close to experimental calorific values. To generate the heat energy it is preferable to burn the biomass waste supplemented with the waste plastic, since such combustion technology provides more thermal energy than separate firing of biomass, as well as less emission of carbon dioxide in comparison with separate burning of plastic waste only.
References
- Ioelovich M. Problems of solid biofuels made of plant biomass. Adv Energy. 2014;2(1):15-20.
- Energy recover from waste. Report of New Energy Co. New Energy, West Perth, 2016.
- SurisAL. Heat of combustion of compounds. 2007;Chem Pertol Eng.2007;43(1-2):20-1.
- ParikhJ. Channiwala SA, Ghosal GK. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel. 2005;84:487-94.
- WaltersRN, Lyon RE, HackettSM. Heats of combustion of high-temperature polymers. Fire and Mater.2000;24:1-13.
- HuggettC.Estimation of rate of heat release by means of oxygen consumption measurements. Fire and Mater.1980;4:61-5.
- Babrauskas V. Heat Release in Fires. Ch.8. Elsevier, London.1992.
- Ioelovich M. Study of thermodynamic stability of various allomorphs of cellulose. J Basic Appl Res Int. 2016;16(2):96-103.