Original Article
, Volume: 13( 2)Comparative Analysis of Chemical Composition of Three Elsholtzia Volatile Oils
- *Correspondence:
- Ganpeng L, Key Laboratory of Chemistry in Ethnic Medicinal Resouces, State Ethnic Affairs Commission and Ministry of Education, Yunnan Minzu University, Kunming 650504, China, Tel: 0086 087165936602; E-mail: ganpeng_li@sina.com
Received: August 10, 2017; Accepted: November 17, 2017; Published: November 22, 2017
Citation: Sheng L, Linyun M, Mingfeng W, et al. Comparative Analysis of Chemical Composition of Three Elsholtzia Volatile Oils. Nat Prod Ind J. 2017;13(2):113
Abstract
Objective: Volatiles oils from three Elsholtzia plants (E. capituligera C.Y.Wu, E. fruticosa (D.Don) Rehd. and E. bodinieri Vaniot) were extracted by simultaneous distillation extraction device (SDE). Their volatile compounds were isolated and characterized by GC-MS, in order to provide a theoretical basis for its further development and utilization.
Methods: Under the same conditions, the main volatile compounds were identified by WILEY and NIST and the relative percentage of volatile oil were determined by using the normalization method.
Results: The main volatile compounds for each species were Eucalyptol (Relative content: 31.55%), (-)-Verbenone (Relative content: 25.36%) and 2, 5-dimethyl-2-Isopropenyl-1-cyclohexanone (Relative content:12.26%) in Elsholtzia capituligera C.Y.Wu; 2, 5-dimethyl-2-Isopropenyl-1-cyclohexanone (Relative content: 60.78%) and (-)-Verbenone (Relative content: 19.78%) in Elsholtzia Fruticosa (D.Don) Rehd; Eucalyptol (Relative content: 55.08%) and Terpinyl acetate (Relative content: 5.64%) in Elsholtzia bodinieri Vaniot.
Conclusion: Comparing of volatile compounds of these three samples showed that the volatiles oil of these genus plants have some differences, the reason might be determined by genotype. This study not only provides a lot of valuable experimental data, but also greatly promoted the volatiles oil research for other researchers.
Keywords
Elsholtzia capituligera C.Y.Wu; Elsholtzia fruticosa (D.Don) Rehd.; Elsholtzia bodinieri vaniot; Gas chromatography-mass spectrometry (GC-MS); Volatile oil
Introduction
The genus Elsholtzia willd (Laminaceae) is mainly distributed in eastern Asia, of which three species distributed in Ethiopia in Africa, and only one was found in extension to Europe and North America. It is reported that this genus has about 40 species, of which 33 species, 15 varietals and 5 forma are distributed in China [1]. In china 26 species and 11 varietals could be found in Yunnan Province [2]. Many species of this genus have been used as Traditional Chinese Medicine for a long time. The volatile oil of Elsholtzia exhibit some beneficial pharmacological effects, such as analgesia, sedation, antispasmodic, antibacterial, anti-inflammatory and antioxidant [3-7]; and could be used in natural air fresheners, food preservation, food additives, rare wild vegetable oil and other fields [8,9].
E. capituligera C.Y.Wu, mainly distributed in the northwest of Yunnan Province and southwest of Sichuan Province, growsin the weathered gravel with dry and abundant sunshine at elevations of 2000 meters to 3000 meters [10]. In the Tibetan medicine system, the growth of a year of foliage and inflorescence medicine has been used to treat anal, fetal, skin, and gastrointestinal diseases and topical anti-mosquito bites.
E. fruticosa (D.Don) Rehd., mainly distributed in the south of Gansu Province (Bailongjiang River Basin), western of HubeiProvince, Sichuan Province, Tibet Autonomous Region, Yunnan Province, Guizhou Province and Guangxi Province, and Bhutan, Nepal, Sikkim, Northern India, grows in the valley side, bottom, roadside, hillside and grass at elevations of 1200-3200 meters. It has been used to the treatment of rash and detoxification [11].
E. bodinieri Vaniot, mainly distributed in the southwest, western, central and southern of Yunnan province, grows in theslopes and in a drier environment at elevations of 1200 meters to 3000 meters. It has been used to treat the headache fever, toothache, sore throat, diarrhea, indigestion, eye pain, urinary and hepatitis [12]. To the best of our knowledge, Elsholtiza bodinieri Vaniot is the only edible plant in this genus [13].
Materials and Methods
Herbs
The whole plant of E. capituligera C.Y.Wu was collected from the Lancang River Basin RUMEI power station in Mangkang County, Tibet Autonomous Region, May, 2016 (Specimen number: YNNI16-05-11). E. fruticosa (D.Don) Rehd. was collected from the Lancang River Valley 318 line (La Wu village) in Mangkang County, Tibet Autonomous Region, May, 2016 (Specimen number: YNNI16-05-12). E. bodinieri Vaniot was collected from Yuxi City Xinping County, July, 2016 (Specimen number: YNNI16-07-18). The plant samples were identified by Dr. Yang Lipan from Yunnan College of traditional Chinese medicine. The samples were stored in the Key Laboratory of Chemistry in Ethnic Medicinal Resouces, State Ethnic Affairs Commission and Ministry of Education, Yunnan Minzu University. All these herbs are air-dried.
Reagents
All reagents were analytical grade. Dichloromethane, sodium chloride and anhydrous sodium sulfate were obtained from the Tianjin Fengchuan Chemical Reagents Technology Co., Ltd. Distilled water was purchased from Watsons.
Instruments and equipment
Simultaneous Distillation Extraction Device was homemade; PE Clarus 600 Gas Chromatography Mass Spectrometer with Electron Impact Ion Source (EI) and WILEY, NIST Mass Spectrometry Database were from PerkinElmer, USA; DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm) was produced by the United States Agilent; one thousandth of the electronic analytical balance was obtained from the Austrian Hauser Instruments Shanghai Co., Ltd.; KDM-type adjustable temperature heating sets 250 mL, 2000 mL was produced by Shandong Juancheng Hualu Electric Instrument Co., Ltd. Rotary Evaporator was purchased from Shanghai Ailang Instrument Co., Ltd.
Test conditions
Gas chromatographic conditions: Column: DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm), inlet temperature of 250°C. Temperature program: the initial temperature of 50°C and keep 5 min, then to 2°C/min the heating rate of up to 80°C, then to 3°C/min the heating rate of up to 230°C and keep the temperature of 16 min, then to 3°C/min the heating rate of up to 250°C and keep the temperature of 20 min. The final set helium flow rate of 0.7 mL/min, injection volume of 2.0 μL and adjust the gas flow rate of 30: 1.
Mass spectrometry conditions: Ion source is electron bombardment (EI) at 180°C, and the ionization energy is 70 eV. Transmission line temperature is 260°C. The monitoring mode is full ion scanning, and the range of mass scan is m/z35 ~ 400. The solvent delay time is 5 min.
Experimental Content
Pretreatment of herbs
Dried herbs were cut into small pieces of 2 ~ 3 cm, spare.
Simultaneous distillation extraction of volatile components from herbs
The pre-processed herbs (10.0 g) were treated by simultaneous distillation extraction method for 4 hours with 300 mL of distilled water (dissolved in 30.0 g sodium chloride, the purpose is to reduce the volatile oil components dissolved in water) and 50 mL of dichloromethane. Then the samples were cooled to room temperature, followed by extracting with methylene chloride and dichloromethane together. The anhydrous sodium sulfate was used to remove water from the extract. Finally, the yellow oil was collected after filtration and distillation, which was stored in sealed vials in a refrigerator at 0°C before analysis.
The relative content of volatile oil were determined
The volatile oil is a complex mixture, and its boiling point is generally between 70~300°C. Therefore, the relative content of volatile oil was determined by GC-MS with peak area normalization method.
Calculation formula : 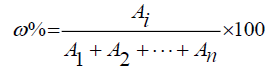
Note: “Ai” means Peak area. “ω%” means relative content.
Ingredient identification
The total ion chromatogram of the volatile components was analyzed as following (Figure 1 to Figure 3) under the conditions of the gas chromatographic mass spectrometry (GC-MS) given above.
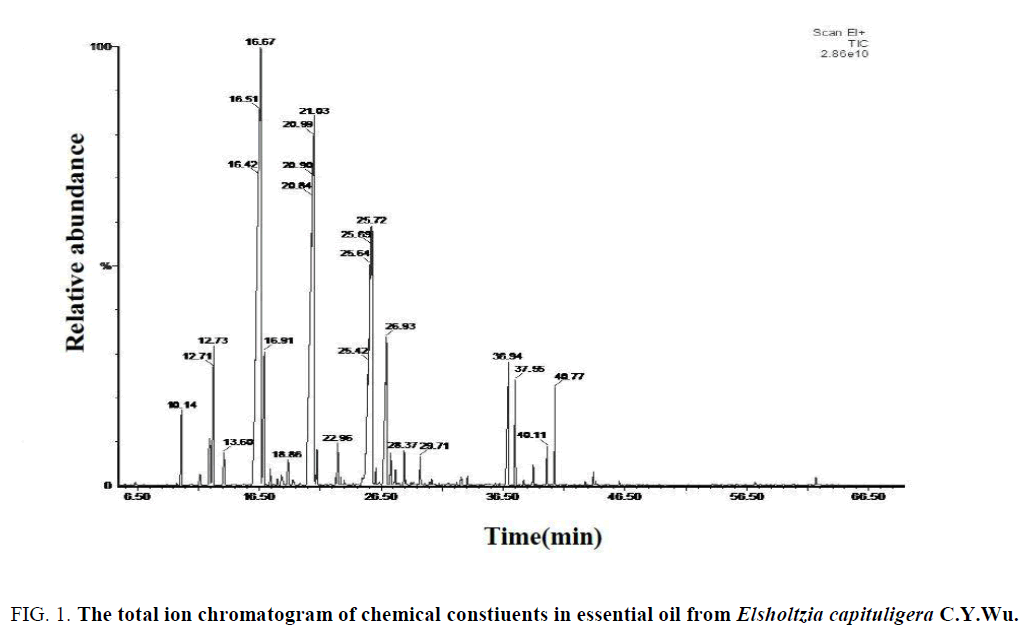
Figure 1: The total ion chromatogram of chemical constiuents in essential oil from Elsholtzia capituligera C.Y.Wu.
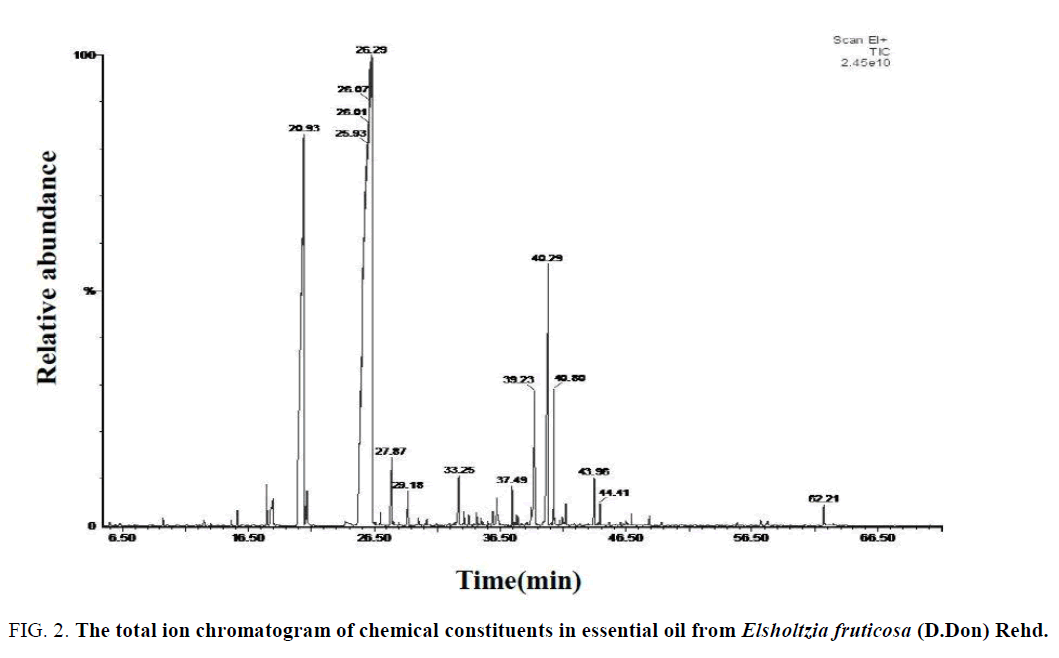
Figure 2: The total ion chromatogram of chemical constituents in essential oil from Elsholtzia fruticosa (D.Don) Rehd.
Figure 3: The total ion chromatogram of chemical constituents in essential oil from Elsholtiza bodinieri Vaniot.
According to the WILEY and NIST Mass Spectrometry Database, the main chemical constituents of these three volatile oil were retrieved (Table 1) and the relative percentage of each compound was calculated by initial quantification.
| Serial No. | Name | CAS no. | Formula | E. capituligera C.Y.Wu | E. fruticosa (D.Don) Rehd. | E. bodinieri Vaniot | |||
|---|---|---|---|---|---|---|---|---|---|
| RT (minutes) | RC (%) | RT (minutes) | RC (%) | RT (minutes) | RC (%) | ||||
| 1 | Furfural | 98-01-1 | C5H4O2 | 5.5 | 0.01 | 5.49 | 0.04 | 5.49 | 0.13 |
| 2 | 2-Hexenal | 505-57-7 | C6H10O | 6.32 | 0.03 | - | - | - | - |
| 3 | (E)-4-Hexen-1-ol | 928-92-7 | C6H12O | 6.42 | 0.01 | - | - | - | - |
| 4 | 5-methyl-2-Furancarboxaldehyde | 620-02-0 | C6H6O2 | 8.92 | 0.01 | 8.92 | 0.02 | - | - |
| 5 | 4-methyl-1-(1-methylethyl)-Bicyclo[3.1. 0] hexane didehydro deriv. | 58037-87-9 | C10H16 | 9.75 | 0.03 | 9.75 | 0.08 | 9.76 | 0.21 |
| 6 | (1R)-(+)-α-pinene | 7785-70-8 | C10H16 | 10.14 | 1.19 | - | - | 10.16 | 1.87 |
| 7 | 4, 4-Dimethyl-2-buten-4-olide | 20019-64-1 | C6H8O2 | 10.87 | 0.01 | 10.84 | 0.03 | 10.94 | 0.37 |
| 8 | Camphene | 79-92-5 | C10H16 | 10.99 | 0.03 | - | - | 11 | 0.26 |
| 9 | Benzaldehyde | 100-52-7 | C7H6O | 11.64 | 0.22 | 11.62 | 0.02 | - | - |
| 10 | 4-methylene-1-(1-methylethyl)-Bicyclo [3.1.0] hexane | 3387-41-5 | C10H16 | 12.46 | 1.12 | 12.39 | 0.02 | 12.48 | 1.33 |
| 11 | Myrcene | 123-35-3 | C10H16 | 13.61 | 0.67 | 13.54 | 0.02 | - | - |
| 12 | Pseudolimonene | 499-97-8 | C10H16 | 14.34 | 0.01 | - | - | - | - |
| 13 | 3-Methylene-1, 5, 5-trimethylcyclohexene | 16609-28-2 | C10H16 | 15.24 | 0.04 | - | - | - | - |
| 14 | Eucalyptol | 470-82-6 | C10H18O | 16.67 | 31.55 | - | - | 16.8 | 55.08 |
| 15 | (E)-3,7-Dimethyl-1,3,6-octatriene | 3779-61-1 | C10H16 | 16.91 | 2.57 | - | - | - | - |
| 16 | Benzeneacetaldehyde | 122-78-1 | C8H8O | 17.04 | 0.02 | 16.93 | 0.03 | - | - |
| 17 | 3, 7-dimethyl-1, 3, 6-Octatriene | 13877-91-3 | C10H16 | 17.41 | 0.21 | - | - | - | - |
| 18 | γ-Terpinene | 99-85-4 | C10H16 | 18.03 | 0.1 | 18 | 0.67 | 18.06 | 0.32 |
| 19 | Acetophenone | 98-86-2 | C8H8O | 18.31 | 0.28 | 18.43 | 0.81 | - | - |
| 20 | cis-4-Thujanol | 15537-55-0 | C10H18O | 18.86 | 0.64 | 18.86 | 0.03 | 20.93 | 0.17 |
| 21 | α-Pinene epoxide | 1686-14-2 | C10H16O | 19.26 | 0.13 | - | - | - | - |
| 22 | (+)-4-Carene | 29050-33-7 | C10H16 | 19.83 | 0.05 | - | - | - | - |
| 23 | (-)-Verbenone | 1196-01-6 | C10H14O | 21.03 | 25.36 | 20.93 | 19.78 | 27.24 | 0.4 |
| 24 | Perillene | 539-52-6 | C10H14O | 21.14 | 0.07 | 21.05 | 0.11 | - | - |
| 25 | Linalool | 78-70-6 | C10H18O | 21.23 | 0.4 | 21.15 | 0.34 | 21.02 | 0.18 |
| 26 | 2-Ethenyl-1, 1-dimethyl-3-methylenecyclohexane | 95452-08-7 | C11H18 | 21.97 | 0.01 | - | - | - | - |
| 27 | trans-p-Mentha-2,8-dienol | - | C10H16O | 22.27 | 0.03 | - | - | - | - |
| 28 | cis-1-methyl-4-(1-methylethyl)-2-Cyclohexen-1-ol | 29803-82-5 | C10H18O | 22.41 | 0.02 | - | - | - | - |
| 29 | (4E,6Z)-2, 6-Dimethyl-2, 4, 6-octatriene | 7216-56-0 | C10H16 | 22.81 | 0.08 | - | - | - | - |
| 30 | 1, 4-dimethyl-Bicyclo[2.1.0]pentane | 17065-18-8 | C7H12 | 22.96 | 0.56 | - | - | - | - |
| 31 | Limonene 1, 2-epoxide | 1195-92-2 | C10H16O | 23.21 | 0.11 | - | - | - | - |
| 32 | Pinocarveol | 5947-36-4 | C10H16O | 23.33 | 0.01 | - | - | - | - |
| 33 | 3,7 -dimethyl-1,7-octadien-3-ol | 598-07-2 | C10H18O | 23.49 | 0.06 | - | - | - | - |
| 34 | 2-(2-Propenyl) bicyclo[2.2.1]heptane | 2633-80-9 | C10H16 | 24.17 | 0.06 | 24.18 | 0.29 | - | - |
| 35 | 1-(2-furanyl)-3-methyl-2-Butanone | 20907-04-4 | C9H12O2 | 24.48 | 0.04 | - | - | - | - |
| 36 | 2, 6-Dimethyl-1-nonen-3-yn-5-ol | - | C11H18O | 25.43 | 2.38 | - | - | - | - |
| 37 | 2, 5-dimethyl-2-Isopropenyl-1-cyclohexanone | 6711-26-8 | C11H18O | 25.72 | 12.26 | 25.29 | 60.78 | - | - |
| 38 | (-)-4-Terpineol | 20126-76-5 | C10H18O | 26.04 | 0.15 | - | - | - | - |
| 39 | (E)-2, 6-Dimethyl-3,7-octadiene-2,6-diol | 13741-21-4 | C10H18O2 | 26.37 | 0.07 | - | - | - | - |
| 40 | α-Terpineol | 98-55-5 | C10H18O | 26.93 | 4.06 | 26.94 | 0.02 | - | - |
| 41 | 2, 5-Dimethylfuran | 625-86-5 | C6H8O | 27.7 | 0.22 | 27.87 | 1.11 | - | - |
| 42 | 2-Cyclopentene-1-carboxylic acid, 1, 2-dimethyl-, ethyl este | 5809-03-0 | C10H16O2 | 27.83 | 0.02 | - | - | - | - |
| 43 | 7-methyl-3-methylene-6-octen-1-ol | 13066-51-8 | C10H18O | 27.95 | 0.04 | - | - | - | - |
| 44 | 1, 3, 3-trimethyl-2-Oxabicyclo[2.2.2]octan-6-ol | 18679-48-6 | C10H18O2 | 28.37 | 0.54 | - | - | 28.39 | 0.45 |
| 45 | Citronellol | 106-22-9 | C10H20O | 28.53 | 0.06 | - | - | - | - |
| 46 | (Z)-3, 7-dimethylocta-2, 6-dienal | 106-26-3 | C10H16O | 28.98 | 0.03 | - | - | - | - |
| 47 | (1α, 2β, 5α)-2-Methyl-5-(1-methylvinyl) cyclohexan-1-ol | 38049-26-2 | C10H18O | 29.16 | 0.03 | - | - | - | - |
| 48 | 2, 6-Dimethyl-1,7-octadien-3-ol | 22460-59-9 | C10H18O | 29.52 | 0.02 | - | - | - | - |
| 49 | p-menth-1-en-3-one | 89-81-6 | C10H16O | 29.71 | 0.41 | - | - | - | - |
| 50 | 2-(2-methylpropylidene)-Cyclohexanone | 43108-69-6 | C10H16O | 30.09 | 0.02 | - | - | - | - |
| 51 | (E)-3, 7-dimethyl-2,6-Octadienal | 141-27-5 | C10H16O | 30.51 | 0.03 | - | - | - | - |
| 52 | 3-(1-Methylethenyl)-2, 5-dimethyl-3,4-hexadien-2-ol | 15448-75-6 | C11H18O | 30.65 | 0.07 | 30.68 | 0.07 | - | - |
| 53 | 2-Oxabicyclo[2.2.2]octan-6-ol, 1, 3, 3-trimethyl-, acetate | 57709-95-2 | C12H20O3 | 31.26 | 0.02 | - | - | - | - |
| 54 | Dehydroelsholtzione | 6138-88-1 | C10H12O2 | 33.02 | 0.03 | - | - | - | - |
| 55 | Elixene | 8/8/3242 | C15H24 | 33.56 | 0.11 | 33.6 | 0.15 | - | - |
| 56 | 8-(1-Methylethylidene)bicyclo [5.1.0]octane | 54166-47-1 | C11H18 | 35.36 | 0.02 | - | - | - | - |
| 57 | β-Bourbonene | 5208-59-3 | C15H24 | 35.88 | 0.03 | 35.91 | 0.21 | - | - |
| 58 | β-Elemene | 515-13-9 | C15H24 | 36.21 | 0.02 | 36.24 | 0.39 | - | - |
| 59 | Methyl eugenol | 93-15-2 | C11H14O2 | 36.94 | 3.05 | - | - | - | - |
| 60 | β-Caryophyllene | 87-44-5 | C15H24 | 37.55 | 1.63 | 37.49 | 0.54 | 37.48 | 0.38 |
| 61 | 1-Cyclopropyl-1-propanone | 6704-19-4 | C6H10O | 38.19 | 0.06 | - | - | 38.33 | 0.97 |
| 62 | 1, 1, 7-Trimethyl-4-methylenedecahydro-1H-cyclopropa[e]azulene? | 25246-27-9 | C15H24 | 38.54 | 0.01 | - | - | 39.16 | 0.12 |
| 63 | α-Caryophyllene | 6753-98-6 | C15H24 | 39.01 | 0.26 | - | - | 39 | 0.26 |
| 64 | (+)-Aromadendrene | 489-39-4 | C15H24 | 39.17 | 0.01 | - | - | - | - |
| 65 | Germacrene D | 23986-74-5 | C15H24 | 40.11 | 0.52 | 40.29 | 6.1 | - | 0.23 |
| 66 | (+)-Ledene | 21747-46-6 | C15H24 | 40.51 | 0.01 | - | - | - | - |
| 67 | α-Farnesene | 502-61-4 | C15H24 | 41.2 | 0.01 | 41.22 | 0.07 | - | - |
| 68 | Thymoquinone | 490-91-5 | C10H12O2 | 42.81 | 0.01 | - | 0.03 | - | - |
| 69 | Geranyl isobutyrate | 2345-26-8 | C14H24O2 | 43.26 | 0.04 | - | - | - | - |
| 70 | Spathulenol | 77171-55-2 | C15H24O | 43.91 | 0.17 | 46.06 | 0.04 | 43.96 | 0.26 |
| 71 | Caryophyllene oxide | 1139-30-6 | C15H24O | 44.09 | 0.06 | - | - | 44.14 | 0.81 |
| 72 | Globulol | 51371-47-2 | C15H26O | 44.24 | 0.01 | 44.26 | 0.02 | 44.28 | 0.08 |
| 73 | n-Hexadecanoic acid | 57-10-3 | C16H32O2 | 57.6 | 0.01 | 57.72 | 0.08 | - | - |
| 74 | Phytol | 150-86-7 | C20H40O | 62.19 | 0.09 | 62.21 | 0.24 | - | - |
| 75 | Diisooctyl phthalate | 27554-26-3 | C24H38O4 | 74.54 | 0.02 | 74.56 | 0.02 | - | - |
| 76 | 1-Acetyl-2-methylcyclopentene | 3168-90-9 | C8H12O | - | - | 5.98 | 0.02 | - | - |
| 77 | Furfuryl alcohol | 98-00-0 | C5H6O2 | - | - | 6.31 | 0.04 | - | - |
| 78 | Mushroom alcohol | 3391-86-4 | C8H16O | - | - | 13 | 0.09 | - | - |
| 79 | Terpinolene | 586-62-9 | C10H16 | - | - | 15.17 | 0.09 | - | - |
| 80 | 1-isopropyl-2-methylbenzene | 527-84-4 | C10H14 | - | - | 15.68 | 0.21 | 15.71 | 0.37 |
| 81 | Dipentene | 138-86-3 | C10H16 | - | - | 15.98 | 0.02 | - | - |
| 82 | 2-Methylbutyl 2-methylbutyrate | 2445-78-5 | C10H20O2 | - | - | 21.34 | 0.02 | - | - |
| 83 | Octen-1-ol acetate | 32717-31-0 | C10H18O2 | - | - | 21.67 | 0.02 | 39.76 | 0.28 |
| 84 | 2-(4-Methylphenyl)propan-2-ol | 1197-01-9 | C10H14O | - | - | 26.62 | 0.02 | 26.29 | 0.51 |
| 85 | Cuminaldehyde | 122-03-2 | C10H12O | - | - | 27.03 | 0.13 | 29.06 | 0.11 |
| 86 | Benzylacetone | 2550-26-7 | C10H12O | - | - | 29.18 | 0.46 | - | - |
| 87 | Benzalacetone | 122-57-6 | C10H10O | - | - | 29.85 | 0.02 | - | - |
| 88 | 4-Phenyl-2-butanol | 2344-70-9 | C10H14O | - | - | 30.02 | 0.08 | - | - |
| 89 | Lavandulol, acetate | - | C12H20O2 | - | - | 31.46 | 0.03 | - | - |
| 90 | (E)-Benzalacetone | 1896-62-4 | C10H10O | - | - | 34.67 | 0.17 | - | - |
| 91 | nerol acetate | 141-12-8 | C12H20O2 | - | - | 34.96 | 0.07 | 35.87 | 0.21 |
| 92 | α-Copaene | 3856-25-5 | C15H24 | - | - | 35.56 | 0.06 | 35.58 | 0.17 |
| 93 | α-Gurjunene | 489-40-7 | C15H24 | - | - | 36.95 | 0.02 | - | - |
| 94 | β-Cubebene | 13744-15-5 | C15H24 | - | - | 37.92 | 0.08 | - | - |
| 95 | 2-methylene-5-(1-methylvinyl)-8-methyl-Bicyclo[5.3.0]decane | - | C15H24 | - | - | 38.27 | 0.02 | - | - |
| 96 | (E)-β-Farnesene | 18794-84-8 | C15H24 | - | - | 39.23 | 2.24 | - | - |
| 97 | (-)-Isoledene | - | C15H24 | - | - | 40.56 | 0.03 | - | - |
| 98 | γ-Elemene | 339154-91-5 | C15H24 | - | - | 40.8 | 1.91 | 50.06 | 0.09 |
| 99 | γ-Cadinene | 39029-41-9 | C15H24 | - | - | 41.44 | 0.07 | - | - |
| 100 | (-)-β-Cadinene | 523-47-7 | C15H24 | - | - | 41.68 | 0.22 | - | - |
| 101 | Dihydroactindiolide | 15356-74-8 | C11H16O2 | - | - | 41.81 | 0.02 | - | - |
| 102 | 1, 2, 4a, 5, 6, 8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl) naphthalene | 483-75-0 | C15H24 | - | - | 42.37 | 0.03 | - | - |
| 103 | Nerolidol | 40716-66-3 | C15H26O | - | - | 43.46 | 0.02 | - | - |
| 104 | 1-Hydroxy-1, 7-dimethyl-4-isopropyl-2, 7-cyclodecadiene | 72120-50-4 | C15H26O | - | - | 43.96 | 0.64 | - | - |
| 105 | β-Eudesmol | 473-15-4 | C15H26O | - | - | 44.41 | 0.23 | - | - |
| 106 | Ledene oxide-(II) | - | C15H24O | - | - | 46.29 | 0.02 | - | - |
| 107 | T-Cadinol | - | C15H26O | - | - | 46.44 | 0.06 | - | - |
| 108 | α-Cadino | 481-34-5 | C15H26O | - | - | 46.94 | 0.15 | 46.96 | 0.25 |
| 109 | (+)-Carotol | 465-28-1 | C15H26O | - | - | 48.32 | 0.13 | 48.33 | 0.22 |
| 110 | Myristinaldehyde | 124-25-4 | C14H28O | - | - | 49.29 | 0.04 | - | - |
| 111 | Perhydrofarnesyl acetone | 502-69-2 | C18H36O | - | - | 53.71 | 0.02 | 53.72 | 0.31 |
| 112 | cis, cis, cis-7,10,13-Hexadecatrienal | 56797-43-4 | C16H26O | - | - | 55.31 | 0.04 | - | - |
| 113 | Geranyl linalool | 1113-21-9 | C20H34O | - | - | 57.16 | 0.1 | - | - |
| 114 | Linolenic acid | 463-40-1 | C18H30O2 | - | - | 62.95 | 0.02 | - | - |
| 115 | n-Octacosane | 630-02-4 | C28H58 | - | - | 73.8 | 0.03 | - | - |
| 116 | n-Eicosane | 112-95-8 | C20H42 | - | - | 83.4 | 0.03 | - | - |
| 117 | rans-1-Ethoxy-1-butene | - | C6H12O | - | - | - | - | 5.99 | 0.14 |
| 118 | Leaf aldehyde | 6728-26-3 | C6H10O | - | - | - | - | 6.33 | 0.08 |
| 119 | Acetonyl acetate | 592-20-1 | C5H8O3 | - | - | - | - | 6.84 | 0.09 |
| 120 | 1-Nonen-4-yne | 31508-12-0 | C9H14 | - | - | - | - | 7.5 | 0.13 |
| 121 | 4-methylene-1-(1-methylethyl)-Bicyclo [3.1.0] hex-2-ene | 36262-09-6 | C10H14 | - | - | - | - | 11.24 | 0.1 |
| 122 | β-Pinene | 127-91-3 | C10H16 | - | - | - | - | 12.77 | 4.79 |
| 123 | Dehydrocineole | 92760-25-3 | C10H16O | - | - | - | - | 13.5 | 0.59 |
| 124 | (1S)-(1)-β-Pinene | 18172-67-3 | C10H16 | - | - | - | - | 14.36 | 0.12 |
| 125 | α-Terpinene | 99-86-5 | C10H16 | - | - | - | - | 15.24 | 0.18 |
| 126 | cis-5-Ethenyltetrahydro-α,α-5-trimethyl-2-furanmethanol | 5989-33-3 | C10H18O2 | - | - | - | - | 18.91 | 0.57 |
| 127 | (E)-Linalool oxide | 34995-77-2 | C10H18O2 | - | - | - | - | 20 | 0.1 |
| 128 | 1-methyl-4-(1-methylethenyl)-benzen | 1195-32-0 | C10H12 | - | - | - | - | 20.13 | 0.12 |
| 129 | 2-Pinen-4-one | 80-57-9 | C10H14O | - | - | - | - | 20.6 | 3.18 |
| 130 | 7, 7-Dimethyl-bicyclo[2.2.1]heptan-2-ol | 26908-71-4 | C9H16O | - | - | - | - | 21.31 | 0.08 |
| 131 | 3, 3, 6-trimethyl-1, 5-Heptadien-4-ol | 27644-04-8 | C10H18O | - | - | - | - | 21.85 | 0.12 |
| 132 | Fenchol | 1632-73-1 | C10H18O | - | - | - | - | 22.05 | 0.08 |
| 133 | trans-1-methyl-4-(1-methylethyl)-2-Cyclohexen-1-ol | 29803-81-4 | C10H18O | - | - | - | - | 22.41 | 0.13 |
| 134 | Campholenic aldehyde | 4501-58-0 | C10H16O | - | - | - | - | 22.51 | 0.12 |
| 135 | (1R)-(+)-Nopinone | 38651-65-9 | C9H14O | - | - | - | - | 23.17 | 0.79 |
| 136 | (-)-Trans-pinocarveol | 547-61-5 | C10H16O | - | - | - | - | 23.42 | 1.74 |
| 137 | (S)-cis-Verbenol | 18881-04-4 | C10H16O | - | - | - | - | 23.76 | 0.43 |
| 138 | 5-(1-methylethyl)-Bicyclo[3.1.0] hexan-2-one | 513-20-2 | C9H14O | - | - | - | - | 24.41 | 0.45 |
| 139 | Pinocarvone | 30460-92-5 | C10H14O | - | - | - | - | 24.65 | 0.75 |
| 140 | (-)-α-Terpineol | 10482-56-1 | C10H18O | - | - | - | - | 25.27 | 0.87 |
| 141 | 2, 2, 6-Trimethyl-6-ethenyltetrahydro-2H-pyran-3-ol | 14049-11-7 | C10H18O2 | - | - | - | - | 25.6 | 0.1 |
| 142 | 3-acetoxy-4-(1-hydroxy-1-methylethyl)-1-methyl-Cyclohexene | - | C12H20O3 | - | - | - | - | 25.35 | 0.21 |
| 143 | Terpinen-4-ol | 562-74-3 | C10H18O | - | - | - | - | 25.82 | 0.89 |
| 144 | Myrtenal | 564-94-3 | C10H14O | - | - | - | - | 26.58 | 0.87 |
| 145 | Myrtenol | 515-00-4 | C10H16O | - | - | - | - | 26.81 | 2.4 |
| 146 | Decanal | 112-31-2 | C10H20O | - | - | - | - | 27.4 | 0.08 |
| 147 | cis-2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-ol | 1197-06-4 | C10H16O | - | - | - | - | 28 | 0.44 |
| 148 | 2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-one | 99-49-0 | C10H14O | - | - | - | - | 29.19 | 0.41 |
| 149 | Lilac alcohol D | 33081-37-7 | C10H18O2 | - | - | - | - | 29.7 | 0.12 |
| 150 | (1R, 2R, 3S, 5R)-(-)-2, 3-Pinanediol | 22422-34-0 | C10H18O2 | - | - | - | - | 30.66 | 0.17 |
| 151 | 4-(1-methylethyl)-1-Cyclohexene-1-carboxaldehyde | 21391-98-0 | C10H16O | - | - | - | - | 30.83 | 0.09 |
| 152 | Decyl alcohol | 112-30-1 | C10H22O | - | - | - | - | 30.9 | 0.08 |
| 153 | 4-(1-methylethyl)-1, 3-Cyclohexadiene-1-methanol | 1413-55-4 | C10H16O | - | - | - | - | 31.2 | 0.12 |
| 154 | L-Borneol acetat | 5655-61-8 | C12H20O2 | - | - | - | - | 31.29 | 0.29 |
| 155 | p-Isopropylbenzyl alcohol | 536-60-7 | C10H14O | - | - | - | - | 31.75 | 0.48 |
| 156 | Perilla alcohol | 536-59-4 | C10H16O | - | - | - | - | 32.07 | 0.26 |
| 157 | Carvacrol | 499-75-2 | C10H14O | - | - | - | - | 32.23 | 0.1 |
| 158 | 3, 7-dimethyl-2, 6-Octadienoic acid methyl ester | 2349-14-6 | C11H18O2 | - | - | - | - | 33.19 | 0.47 |
| 159 | 4-(1-methylethyl)-1, 4-Cyclohexadiene-1-methanol | 22539-72-6 | C10H16O | - | - | - | - | 33.56 | 0.42 |
| 160 | trans-3-Caren-2-ol | — | C10H16O | - | - | - | - | 34.06 | 0.08 |
| 161 | Terpinyl acetate | 80-26-2 | C12H20O2 | - | - | - | - | 34.48 | 5.64 |
| 162 | decanyl acetate | 112-17-4 | C12H24O2 | - | - | - | - | 37.27 | 0.36 |
| 163 | α-muurolene | 10208-80-7 | C15H24 | - | - | - | - | 40.86 | 0.08 |
| 164 | δ-Cadinene | 483-76-1 | C15H24 | - | - | - | - | 41.66 | 0.18 |
| 165 | d-8-Acetoxycarvotanacetone | 86421-35-4 | C12H18O3 | - | - | - | - | 43.42 | 0.52 |
| 166 | Ledol | 577-27-5 | C15H26O | - | - | - | - | 44.61 | 0.12 |
| 167 | Himbaccol | 552-02-3 | C15H26O | - | - | - | - | 45.02 | 0.65 |
| 168 | Humulene oxide II | 19888-34-7 | C15H24O | - | - | - | - | 45.21 | 0.39 |
| 169 | Isoaromadendrene epoxide | - | C15H24O | - | - | - | - | 46.07 | 0.14 |
| 170 | 11-Hexadecyn-1-ol | 65686-49-9 | C16H30O | - | - | - | - | 47.76 | 0.15 |
| 171 | cis-Z-α-Bisabolene epoxide | - | C15H24O | - | - | - | - | 49.32 | 0.15 |
Table 1: Chemical constituents of volatile oils.
Analysis of the chemical constituents of the volatile oil of E. capituligera C.Y.Wu showed that 76 volatile compounds were isolated and identified. E. fruticosa (D.Don) Rehd. was isolated and identified 71 volatile compounds, and the E. bodinieri Vaniot was isolated and identified 85 volatile compounds. In total, 171 chemical constituents were identified, including 11 identical volatile compounds which were characterized in these three plants at the same time (Serial number of Table: 1, 5, 7, 10, 18, 20, 23, 25, 60, 70, 72), which was accounted for 18.38% of total volatile compounds.
29 identical volatile compounds were discovered in E. capituligera C.Y.Wu and E. fruticosa (D.Don) Rehd. (Serial number of Table: 1, 4, 5, 7, 9, 10, 11, 16, 18, 19, 20, 23, 24, 25, 34, 37, 40, 41, 52, 55, 57, 58, 60, 65, 70, 72, 73, 74, 75), which was accounted for 72.09% of total volatile compounds. The main volatile compounds was 2, 5-dimethyl-2-Isopropenyl-1-cyclohexanone (Serial number of Table: 37) and (-)-Verbenone (Serial number of Table: 23).
20 identical volatile compounds were discovered in E. capituligera C.Y.Wu and E. bodinieri Vaniot (Serial number of Table: 1, 5, 6, 7, 8, 10, 14, 18, 20, 23, 25, 44, 60, 61, 62, 63, 65, 70, 71, 72), which was accounted for 65.80% of total volatile compounds. The main volatile compounds were Eucalyptol (Serial number of Table: 14).
22 identical volatile compounds were discovered in E. fruticosa (D.Don) Rehd and E. bodinieri Vaniot (Serial number of Table: 1, 5, 7, 10, 18, 20, 23, 25, 60, 65, 70, 72, 80, 83, 84, 85, 91, 92, 98, 108, 109, 111), which was accounted for 18.64% of total volatile compounds. Although these two plants have 22 identical volatile components, their relative content of their major volatile components varies greatly.
Results, Discussion and Conclusion
In this experiment, the volatile oils of three kinds of Elsholtzia genus plants were isolated and identified by GC/MS. It was the first example to identified 171 volatile compounds from the title plants. Volatile compounds mainly included ketones, sesquiterpene hydrocarbons, alcohols, esters, and aldehydes. Through our research data, the main volatile compounds for each species were Eucalyptol (Serial number of Table: 14, Relative content: 31.55%), (-)-Verbenone (Serial number of Table: 23, Relative content: 25.36%) and 2, 5-dimethyl-2-Isopropenyl-1-cyclohexanone (Serial number of Table: 37, Relative content: 12.26%) in Elsholtzia capituligera C.Y.Wu; 2,5-dimethyl-2-Isopropenyl-1-cyclohexanone (Serial number of Table: 37, Relative content: 60.78%) and (-)-Verbenone (Serial number of Table: 23, Relative content: 19.78%) in Elsholtzia fruticosa (D.Don) Rehd; Eucalyptol (Serial number of Table: 14, Relative content: 55.08%) and Terpinyl acetate(Serial number of Table: 161, Relative content: 5.64%) in Elsholtzia bodinieri Vaniot. It is showed that E. capituligera C.Y.Wu and E. fruticosa (D.Don) Rehd. have the same major volatile components were 2,5-dimethyl-2-Isopropenyl-1-cyclohexanone (Serial number of Table: 37) and (-)-Verbenone (Serial number of Table: 23). E. capituligera C.Y.Wu and E. bodinieri Vaniot have the same major volatile components was Eucalyptol (Serial number of Table: 14). Although E. fruticosa (D.Don) Rehd and E. bodinieri Vaniot have 22 identical volatile components, their relative content of their majorvolatile components varies greatly. So the volatiles oil of these genus plants have some differences, the reason might be determined by genotype. This study not only provides a lot of valuable experimental data, but also might promote the volatile oils research for other peoples.
Elsholtzia plants are widely used in traditional Chinese medicine for the anti-virus and broad-spectrum antimicrobial. At thesame time, some of these plants can also be used as food materials and nectar plants [14]. Therefore, the economic utilization and academic research of these genus plants might be increased concerned by this research.
Acknowledgement
This work was financially supported by China Tobacco Yunnan Industrial Co., Ltd (JSZX20151008-52).
References
- Chinese Academy of Sciences Chinese Flora Editorial Board. Flora of China; Science Press: Beijing. 1977;66:336-9.
- Kunming Institute of Botany, Chinese Academy of Sciences. Flora of Yunnan. Science Press: Beijing. 1977;1:714.
- Xiang P, Lou GQ, Wang SY, et al. Analysis of volatite oils in Elsholtzia ciliata and Elsholtzia cypriani and evaluation of their biological activities. Chinese Traditional Patent Medicine. 2017;39:1881-85.
- Liang LX, Li J, Chen LJ. Analysis of volatile oil from Elsholtzia ciliata (Thunb.) Hyland. In: Florescence Stage. GC-MS. Flavour Fragrance Cosmetics. 2015;4:6-8.
- Xu XH, Tang FL, Fan ZD, et al. Observation on spatial antibiotic action of the volatile oil from several Chinese herbs. 2010;19:2618-19.
- Kang SH. Research progress on essential oils and pharmacology of Elsholtzia in China. J Northwest Univ. Natural Science Edition. 2012;33:58-63.
- Pu CX, You C. Comparison of microscopic characters of stem transverse section in Elsholtzia. Lishizhen Medicine and Materia Medica Research. 2013;24:157-160.
- Wu YK, Zhou YL, Zhou SQ, et al. Research on the pharmacological effects of the essential oil from four Elsholtzia plants. Journal of Chinese Medicinal Materials. 1992;15:36-8.
- Zhang ZH, Yin JZ. Advances in pharmacological effects of chemical composition of Elsholtzia and its development and application. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2008;29:48-50.
- Chinese Academy of Sciences Chinese Flora Editorial Board. Flora of China; Science Press: Beijing. 1997;66:319.
- Kang SH, Shi YQ. Supercritical CO2 Fluid Extraction of Elsholtzia Fruticosa and GC/MS Analysis. Journal of Chinese Spectrometry Society. 2009;30:36-40.
- Mao Y, Liu KH, Xu JF, et al. Review of chemical constituents of Elsholtiza Bodinieri Vaniot. Chinese Journal of Information on TCM. 2016;23:134-6.
- Gong MX, Zhu GP. Materia medica textual research of Elsholtzia. Beijing Journal of Traditional Chinese Medicine. 1996;5:39-41.
- Li BY, Zhou XM, Hao FG, et al. Advance and perspectives in studies on Elsholtzia genus. Progress of Elsholtzia Plants in China. Journal of Henan Institute of Science and Technology (Natural Science Edition). 2012;40:37-44.