Original Article
, Volume: 17( 2)Chromatographic Separation of Pesticides from Various Chemical Classes
- *Correspondence:
- Uflyand IE , Department of Chemistry, Southern Federal University, B. Sadovaya Str. 105/42, Rostov-on-Don, 344006, Russia, Tel: +78632199749; E-mail: ieuflyand@sfedu.ru
Received: September 13, 2017; Accepted: September 14, 2017; Published: September 22, 2017
Citation: Voikina AV, Bugayov LA, Uflyand IE. Chromatographic Separation of Pesticides from Various Chemical Classes. Anal Chem Ind J. 2017;17(2):122
Abstract
Features of chromatographic separation of pesticides from various chemical classes by the reversed-phase (RP) high-performance liquid chromatography (HPLC) method under isocratic mode of elution are shown. The most complete separation of the test substances is achieved using a lead electrolyte containing acetonitrile and 0.01 M ortho-phosphoric acid in a ratio of 3:2 (v/v), a flow rate of 0.5 mL min-1 , a column thermostat temperature of 40°C, a detection wavelength of 230 nm, and the volume of the injected sample of 10 μL. The separation time is 35 min. Under these conditions, the values of the chromatographic parameters, in particular, peak resolution (Rs), capacitance factor (k'), asymmetry factor (As), and selectivity coefficient (α), have optimal limits.
Keywords
Pesticides; HPLC; Chromatographic parameters; Eluent; Schatz hydrophobicity parameter
Introduction
At the current stage of development of agricultural production, the range of chemical and biological methods of plant protections is constantly changing. In particular, drugs causing remote environmental consequences are excluded, and a list of useful tools is supplemented by effective compounds with new mechanisms of action in safer preparative forms. In recent years, highly toxic and persistent drugs (mercury-containing, chloroorganic, many organophosphorus, etc.) have been excluded from the list of pesticides used in agriculture [1-5].
Pesticides of the 21st century include sulfonylurea preparations and heterocyclic compounds of various series, in particular, pyridine herbicides, pyrimidine insecticides and fungicides, herbicides based on aryloxyphenoxypropionic acid derivatives, imidazolinone herbicides, insecticides of the phenylpyrazole and neonicotinoide classes, triazole and imidazolinone fungicides. The number of works on the synthesis of these compounds is continuously growing, and they successfully compete in efficiency with previously used pesticides [6].
Along with the use of individual pesticides, their various mixtures are often used in agriculture. Combined pesticides allow simultaneously to destroying weeds, pests and diseases, and are an important reserve for increasing the biological and economic efficiency of chemical methods of plant protection. It is known that the use of combinations of small doses of two or more pesticides can provide the same biological efficacy and duration of action as treatment with a large dose of a more toxic preparation. However, the expansion of simultaneous use of pesticides of various classes leads to a noticeable contamination of soil, surface waters, ground waters and drinking water as well as agricultural products [7,8].
The complexity and diversity of the composition of pesticide preparations, the large number of interfering substances make the control over the pollution of waters by pesticides a very complex analytical task. In addition, the composition of pesticides used varies significantly over time [9]. The search for optimal methods for the analysis of pesticides is one of the most important problems in environmental analytical chemistry. For analytical control of residual amounts of pesticides in agricultural and environmental facilities, methods of chromatography-mass spectrometry and gas chromatography, electrochemical methods, polarography, and enzyme immunoassay are widely used. The method of high-performance liquid chromatography (HPLC) has been used most in routine analyzes; however, the use of toxic substances as solvents and expensive reagents necessitates the selection of appropriate eluents for chromatographic separation and detection [10].
The purpose of this work was to develop optimal conditions for simultaneous chromatographic separation of active substances of pesticides of various chemical classes [11].
Experimental
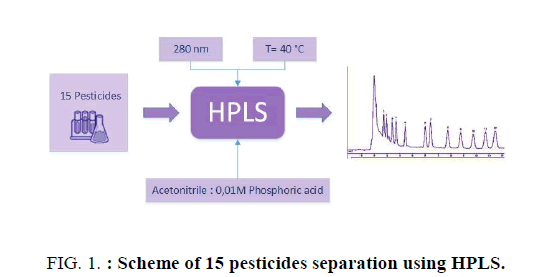
Chromatographic studies were performed on a liquid chromatograph from Applied Biosystems (USA) equipped with a spectrophotometric detector Applied Biosystems Kratos 757 with a deuterium lamp. The operating range is from 190 nm to 360 nm, and the maximum sensitivity is 0.005 AUFS. Isocratic elution of the mobile phase was carried out through a Reprosil-PUR ODS column (4 mm × 150 mm size and 5.0 μm grain size). For the preparation of the mobile phase, acetonitrile purchased from Cryochrome (Russia), bidistilled water and ortho-phosphoric acid were used (Figure. 1). A degasser DG-18 was used to remove air bubbles in the mobile phase. Standard samples of pesticides (>98%) were taken as subjects of study: imazapyr (BASF, Germany), imidacloprid (BASF, Germany), imazethapyr (BASF, Germany), tsiprosulfamid (Bayer CropScience, Germany), metribuzin (Bayer CropScience, Germany), phenmedipham (Bayer CropScience, Germany), flumioxazine (Sumitomo Chemical Co., Japan), quizalofop-P-ethyl (Bayer CropScience, Germany), ethofumesate (Bayer CropScience, Germany), iprodion (BASF, Germany), flufenacet (Bayer Crop-Science, Germany), flubendiamide (Bayer CropScience, Germany), famoxadone (DuPont de Nemours International, Switzerland), pencikuron (Bayer CropScience, Germany), diflufenican (Bayer ?ropScience, Germany). Standard solutions of pesticides with a concentration of 100 μg mL-1 were prepared from dry samples using acetonitrile as the solvent. Calibration solutions of pesticides were stored in the working chamber of the refrigerator at a temperature of +3°C to -5°C in hermetically sealed glass containers for not more than 3 months. Before use, the solutions were kept at room temperature for at least 20 min. Working solutions of a mixture of pesticides were prepared by diluting standard solutions of individual pesticides with acetonitrile immediately before use. Data processing was performed using the MultiChrom v.1.5 software (Ampersand Ltd., USA). The optimal conditions for separation of the components of the separated mixture were determined by the achievement of the following values: the peak resolution Rs ≥ 1.0, the capacitance factor 0.5 ≤ k' ≤ 20, the asymmetry factor 0.7 ≤ As ≤ 1.5 and the selectivity coefficient α ≥ 1.1.
Results and Discussion
When choosing the conditions for chromatographic separation of the mixture of the active substances of pesticides studied, the physical and chemical properties of the separated compounds were taken into account (Table 1). All substances are thermally unstable, low molecular weight aromatic carbocyclic and heterocyclic compounds containing both electronwithdrawing and electron-donating groups as substituents. To assess the degree of hydrophilicity and hydrophobicity of the compounds, the Schatz hydrophobicity parameter (H) and the octanol water partition coefficient (log P) were used, which were calculated by the formulas:
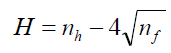 (1)
(1)
| Active substance | Class | Molar mass | ? | log Kow |
|---|---|---|---|---|
| Imazapyr | Imidazolinones | 261.3 | 5 | 0.11 |
| Imidacloprid | Neonicotinoids | 255.7 | 0.6 | 0.57 |
| Imazethapyr | Imidazolinones | 289.3 | 5 | 1.49 |
| Tsiprosulfamid | Methoxybenzamide derivatives | 374.0 | 8 | 0,8 |
| Metribuzin | Triazinones | 214.3 | 0.1 | 1.65 |
| Phenmedipham | Carbamates | 300.3 | 6 | 3.59 |
| Flumioxazine | Phenylphthalimides | 354.3 | 10 | 2.55 |
| Quizalofop-P-ethyl | Aryloxyphenoxypropionates | 372.8 | 9 | 4.61 |
| Ethofumesate | Benzofuranylalkanesulfonates | 286.3 | 5 | 2.7 |
| Iprodion | Dicarboxamides | 330.2 | 5 | 3.1 |
| Flufenacet | Oxyacetanilides | 363.3 | 10 | 3.2 |
| Flubendiamide | Benzenediracarboxamides | 682.4 | 15 | 4.2 |
| Famoxadone | Oxazolidinediones | 374.4 | 12 | 4.8 |
| Pencikuron | Urea derivatives | 328.8 | 13 | 4.68 |
| Diflufenican | Carboxamides | 394.3 | 16 | 4.2 |
Table 1: Brief physicochemical characteristic of the active substances of pesticides.
Where, nh is the number of elementary hydrophobic fragments in the molecule, and nf is the number of polar groups.
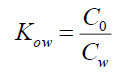 (2)
(2)
Where, Co is the concentration of the test substance in n-octanol saturated with water, and Cw is the concentration of the test substance in water saturated with n-octanol.
All investigated active substances of pesticides belong to low (H=0-4) and medium hydrophobic (H=4-20) substances, which are respectively partially soluble in water and are readily soluble in polar solvents. Given these characteristics, the most effective is the use of a reversed-phase (RP) version of liquid chromatography. A chromatographic column containing a silica-based sorbent chemically modified with alkylsilanes Cl-Si (CH3)2-R was used as a stationary phase, where R is an alkyl chain with eighteen carbon atoms or an octadecylsilated silica gel. A mixture of acetonitrile with a 0.01 M solution of orthophosphoric acid in various ratios (4:1, 7:3, 3:2, 1:1) was used as a mobile phase. Taking into account that the investigated active substances of pesticides are thermally unstable, the temperature of the column thermostat did not exceed 40°C. According to the methodological instructions for determining the residual quantities of individual pesticides, the detector wavelength of 230 nm was chosen. At the given wavelength, the compounds under study give the maximum response. Chromatographic mobility of the investigated active substances of pesticides was studied and the retention time for different composition of the eluent was determined. The results of chromatography in systems with different reagent contents, as an average of five parallel determinations, are presented in Table 2.
| Eluting system | Retention time, min | |||
|---|---|---|---|---|
| ??3CN/ 0.01 ? H3PO4 (4:1) |
??3CN/ 0.01 ? H3PO4 (7:3) |
??3CN/ 0.01 ? H3PO4 (3:2) |
??3CN/ 0.01 ? H3PO4 (1:1) |
|
| Active substance | ||||
| Imazapyr | - | - | 2.735 | 6.861 |
| Imidacloprid | - | - | 2.964 | 6.681 |
| Imazethapyr | - | 2.951 | 3.403 | 9.312 |
| Tsiprosulfamid | 3.234 | 3.032 | 3.705 | 12.012 |
| Metribuzin | - | 4.311 | 4.420 | 13.375 |
| Phenmedipham | 3.876 | 5.304 | 5.957 | 24.645 |
| Flumioxazine | 4.458 | 6.982 | 6.368 | 28.234 |
| Quizalofop-P-ethyl | 5.653 | 7.321 | 7.699 | 32.912 |
| Ethofumesate | 6.234 | 7.512 | 8.714 | 38.042 |
| Iprodion | 7.231 | 8.612 | 9.702 | 43.612 |
| Flufenacet | 8.342 | 9.432 | 10.663 | 45.453 |
| Flubendiamide | 9.054 | 10.234 | 11.425 | 45.567 |
| Famoxadone | 11.321 | 12.241 | 15.008 | 46.921 |
| Pencikuron | 14.765 | 15.442 | 18.245 | 49.925 |
| Diflufenican | 15.076 | 17.453 | 21.243 | - |
Table 2: Retention times of active substances of pesticides with different composition of the mobile phase (n=5, P=0.95).
As seen from the Table 1, the active substances of pesticides with a low Schatz parameter and octanol water partition coefficient are not detected with the eluent containing 80% acetonitrile and 20% 0.01 M ortho-phosphoric acid solution. Imazapyr, imidacloprid, imazethapyr and metribuzin are sorbed irreversibly, i.e. the system has insufficient eluting power with respect to these substances. A decrease in acetonitrile in the mobile phase up to 70% leads to a similar pattern for imazapyr and imidacloprid, while the other active substances of pesticides are separated. With the composition of the mobile phase of 60% acetonitrile and 40% 0.01 M ortho-phosphoric acid solution, all the active substances of the pesticides are separated, and the chromatograms are characterized by fairly narrow peaks. It should be noted that with a further decrease in the volume fraction of acetonitrile in the mobile phase, the time for the release of active substances of pesticides is significantly increased, and for some substances, such as diflufenican, the retention time is not determined.
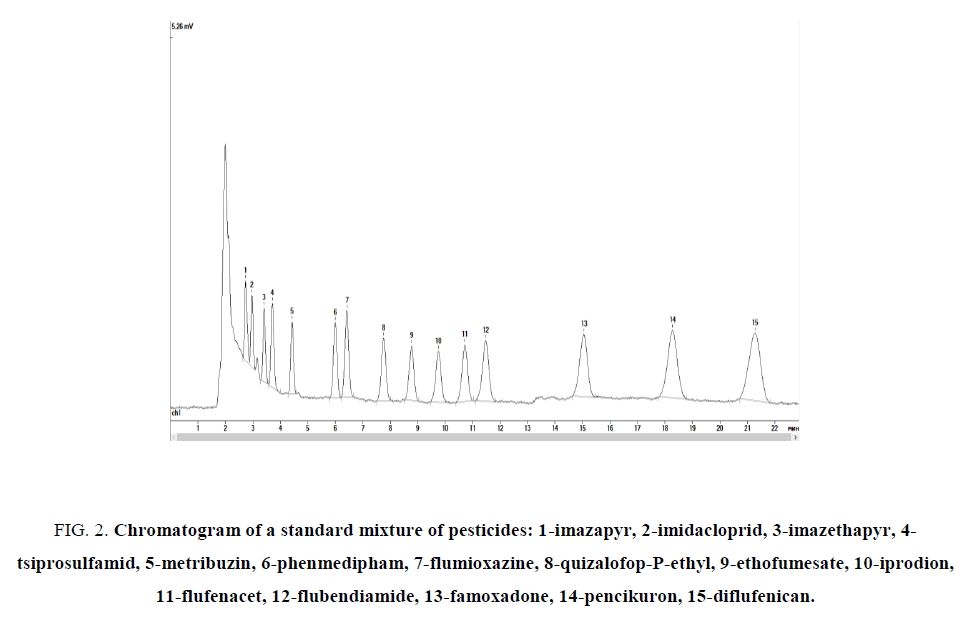
As a result, the complete separation of the test substances was achieved under the following conditions: mobile phase (acetonitrile and 0.01 M ortho-phosphoric acid in a ratio of 3:2 by volume) in isocratic mode; flow rate of 0.5 mL min-1, the temperature of the column thermostat of 40°?, the detection wavelength of 230 nm, the sample volume of 10 μL, the analysis duration of 35 min. Figure. 2 shows a chromatogram of a mixture of standard substances obtained under these conditions.
Figure 2: Chromatogram of a standard mixture of pesticides: 1-imazapyr, 2-imidacloprid, 3-imazethapyr, 4-tsiprosulfamid, 5-metribuzin, 6-phenmedipham, 7-flumioxazine, 8-quizalofop-P-ethyl, 9-ethofumesate, 10-iprodion, 11-flufenacet, 12-flubendiamide, 13-famoxadone, 14-pencikuron, 15-diflufenican.
When choosing the optimal conditions for separation and identification of substances in the mixture, the dependence between the composition of the mobile phase and the chromatographic parameters (Rs-peak resolution, α-selectivity coefficient, As-asymmetry factor, k'-capacitance factor, N-number of theoretical plates). The results of calculation of chromatographic characteristics are presented in Table 3.
| Active substance | Rs | α | As | k' | N |
|---|---|---|---|---|---|
| Imazapyr | 1.48 | 1.3 | 1.68 | 0.57 | 4174 |
| Imidacloprid | 1.11 | 0.59 | 6513 | ||
| 2.79 | 1.5 | ||||
| Imazethapyr | 1.20 | 0.71 | 6619 | ||
| 1.71 | 1.2 | ||||
| Tsiprosulfamid | 1.20 | 0.86 | 6034 | ||
| 3.82 | 1.4 | ||||
| Metribuzin | 1.14 | 1.22 | 8811 | ||
| 7.15 | 1.6 | ||||
| Phenmedipham | 1.01 | 1.99 | 9436 | ||
| 1.65 | 1.1 | ||||
| Flumioxazine | 1.04 | 2.19 | 9691 | ||
| 4.73 | 1.3 | ||||
| Quizalofop-P-ethyl | 0.99 | 2.86 | 9595 | ||
| 3.20 | 1.2 | ||||
| Ethofumesate | 0.93 | 3.37 | 10932 | ||
| 2.89 | 1.1 | ||||
| Iprodion | 0.91 | 3.85 | 11471 | ||
| 2.60 | 1.1 | ||||
| Flufenacet | 0.90 | 4.35 | 11879 | ||
| 1.87 | 1.1 | ||||
| Flubendiamide | 0.91 | 4.71 | 10614 | ||
| 7.34 | 1.4 | ||||
| Famoxadone | 0.85 | 6.52 | 11671 | ||
| 5.30 | 1.2 | ||||
| Pencikuron | 0.95 | 8.16 | 11256 | ||
| 3.99 | 1.2 | ||||
| Diflufenican | 0.83 | 9.64 | 10289 |
Table 3: HPTLC profile of the Nakshatra plants for glycosides.
Under these chromatographic conditions, the peak resolution (Rs) values were in the range from 1.48 to 7.34, indicating a fairly complete separation of the two adjacent components. The values of the capacitance factor (k') for the pesticide active substances studied were from 0.37 to 9.64, which characterizes their retention in the column as optimal. If the selectivity coefficient (α) is ≥ 1.1, the separation is considered complete. In our case, α was in the range from 1.1 to 1.6, which indicates the correct choice of the sorbent in the column and solvent in the mobile phase. The values of the asymmetry factor (As) calculated for the peaks of the investigated compounds were in the range from 0.83 to 1.68 that testifies about the absence of significant ion-exchange interactions. The number of theoretical plates (N) under these chromatographic conditions was in the range from 4174 to 11879, which indicates a large number of steady-state equilibria and high efficiency of the chromatographic column. In the selected optimal chromatographic conditions, calibration characteristics were obtained. The graphs for each active substance of pesticides have a linear relationship with the correlation coefficients R2 ≥ 0.9978 (Table 4).
| Active substance | Retention time, min | Linearity range, μg mL-1 | Equation of calibration graph | Correlation coefficient |
|---|---|---|---|---|
| Imazapyr | 2.70 | 0.03 – 4 | ?=0.022100? | 0.9978 |
| Imidacloprid | 2.93 | 0.08 – 10 | ?=0.081246? | 0.9986 |
| Imazethapyr | 3.36 | 0.04 – 5 | ?=0.039133? | 0.9993 |
| Tsiprosulfamid | 3.66 | 0.05 – 6 | ?=0.036583? | 0.9996 |
| Metribuzin | 4.37 | 0.02 – 3 | ?=0.020694? | 0.9996 |
| Phenmedipham | 5.89 | 0.02 – 3 | ?=0.017073? | 0.9997 |
| Flumioxazine | 6.31 | 0.02 – 2 | ?=0.009263? | 0.9998 |
| Quizalofop-P-ethyl | 7.64 | 0.05 – 6 | ?=0.031069? | 0.9998 |
| Ethofumesate | 8.66 | 0.08 – 10 | ?=0.058973? | 0.9997 |
| Iprodion | 9.65 | 0.06 – 7 | ?=0.041369? | 0.9996 |
| Flufenacet | 10.61 | 0.08 – 10 | ?=0.049935? | 0.9997 |
| Flubendiamide | 11.37 | 0.08 – 10 | ?=0.036661? | 0.9997 |
| Famoxadone | 14.96 | 0.05 – 6 | ?=0.018735? | 0.9998 |
| Pencikuron | 18.22 | 0.06 – 8 | ?=0.017986? | 0.9996 |
| Diflufenican | 21.23 | 0.08 – 10 | ?=0.018329? | 0.9996 |
Table 4: Reported reasons for planned cesarean section in Jordanian women according to sector, 2011-2012.
Conclusion
In conclusion, based on the results of present study, the composition of the mobile phase was selected and optimum elution conditions were found for identification of active substances of pesticides of various chemical classes: imazapyr, imidacloprid, imazethapyr, tsiprosulfamid, metribuzin, phenmedipham, flumioxazine, quizalofop-P-ethyl, ethofumesate, iprodion, flufenacet, flubendiamide, famoxadone, pencikuron, diflufenican, The obtained data indicate that this method ensures complete separation and identification of the investigated pesticides that are simultaneously in the mixture. The developed method of separation of a mixture of pesticides from different classes allows us to proceed to an analysis of the pollution of natural objects with pesticides, which is the subject of our next study.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Ashraf G. Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Plant J. 2011;30(5):351-8.
- Takahashi S, Yoshikawa M, Kamada A, et al. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J Plant Physiol. 2013;170(17):1549-52.
- Ashraf G, Kohnehrouz SB.Identification ofDUF538cDNA clone from Celosia cristata expressed sequences of nonstressed and stressed leaves. RussJPlant Physiol. 2010;57(2):247-52.
- Nakagami H, Sugiyama N, Mochida K, et al. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153(3):1161-74.
- Ashraf G, Kohnehrouz SB. ProtJ.2013;32:163.
- Ashraf G.Prediction of tertiary structure homology between bactericidal/permeability increasing protein of innate immune system and hydrolase enzymes. IntJBiosci. 2014;5(2):1-6.
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seiolman JG, Smith JA, et al. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1991.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402-8.
- Rabbani MA, Maruyama M, Abe H, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant physiol. 2003;133(4):1755-67.
- Shinozaki K, Yamaguchi SK, Seki M, et al. Regulatory network of gene expression in the drought and cold stress responses.CurrOpinPlant Biol. 2003;6(5):410-7.
- Shinozaki K, Yamaguchi SK. Gene networks involved in drought stress response and tolerance. JExpBot. 2007;58(2):221-7.
- Yamaguchi SK, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10(2):88-94.
- Bartels D, Sunkars R. Drought and Salt Tolerance in Plants. CritRevPlant Sci. 2005;24(1):23-58.
- Seki M, Narusaka M, Abe H, et al. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13(1):61-72.
- Schimid M, Davison TS, Hens SR, et al. A gene expression map of Arabidopsis thaliana development. NatGenet. 2005;37(5):501-6.