Original Article
, Volume: 12( 2)Chemical Studies on the Removal of Some Heavy Metals from Industrial Waste Solutions Using Animal Bones and Nile Rose Plant as Natural Sorbents
- *Correspondence:
- Abou-Mesalam MM, Atomic Energy Authority, Hot Labs. Centre, Egypt, Tel: 22876033 22875924; Fax: 22876031; E-mail: mabumesalam@yahoo.com
Received: November 06, 2017; Accepted: December 18, 2017; Published: December 22, 2017
Citation: Abou-Mesalam MM, El-Shorbagy MM, Omran BA, et al. Chemical Studies on the Removal of Some Heavy Metals from Industrial Waste Solutions Using Animal Bones and Nile Rose Plant as Natural Sorbents. Inorg Chem Ind J. 2017;12(2):120.
Abstract
Different types of natural sorbents such as camel bone, cow bone, sheep bone and Nile Rose plant were used for the retention of some toxic heavy metals such as Pb2+, Mn2+, Ni2+ and Cu2+ ions from waste water produced from chemical industry factories using batch technique. Characterizations of the natural sorbents were done using different analytical tool such as X-ray fluorescence and x-ray diffraction spectrometers. Factors affecting on the retention process of the studied toxic cations such as contact time, pH of the medium and the concentration of the metal cations were investigated. The data indicated that the Kd values of Cu2+, Mn2+, Ni2+ and Pb2+ ions are increased with increasing the pH of the solution from 1.0 to 5.0 with the selectivity sequence; Pb2+>Ni2+>Mn2+>Cu2+. Also, the data of isotherm indicated that all the investigated cations were physically sorbed on animal bones and Nile Rose plant sorbents with sorbent surface of heterogeneous type. Finally, column chromatographic was carried out from the results obtained.
Keywords
Removal; Heavy metal; Industrial waste; Animal bones; Nile Rose; Natural sorbents
Introduction
In view of the expectation for increasing of environmental pollution by toxic heavy metals like lead, copper, magnesium and nickel, the need to look for selective materials such as resins, inorganic ion exchange and natural sorbents to extract these metals should be increasing as some trials seen to be promising. The term heavy metals that is in common use, refers to metals with a density greater than a certain value, usually 5 g/cm3 or 6 g/cm3. Natural sorbents have an interest to use to the environmental remediation [1-7], owing to their high capacities [8-10]. Activated carbon is most popular and widely used adsorbent in wastewater treatment throughout the world, but the high prices and regeneration cost of activated carbon limits their largescale use for the removal of inorganic and organic pollutants and has encouraged researchers to look for low cost adsorbing materials. Recently, adsorption of hazardous ions using natural materials or the wastes products from industrial or agricultural operations has emerged as an option for developing economic and eco-friendly wastewater treatment processes. Numerous low-cost adsorbents have so far been studied for the removal of some toxic ions from water and wastewater. Although dry plants and animal bones were very cheap and available in the environment in large scale and can be used as natural sorbents adsorbents, a few works were investigated and mentioned.
In this paper different types of animal bones and Nile Rose plant were used for the decontamination of waste water of the chemical industrial from some of heavy metals such as lead, copper, magnesium and nickel by batch and column techniques. The contact time, hydrogen ion concentration and the concentration of the metal cations were studied. Also, capacity sorption isotherm was evaluated.
Experimental
Materials and reagents
All materials and chemical reagents used are of analytical grade and without further purification. Nile Rose plant was collected from the river around the industrial area and the different samples of animal bones were collected from butcher shops.
Treatment of natural sorbents
Natural sorbents used in this work are different types of animal bones such as camel bone, cow bone and sheep bone and natural plant such as Nile Rose plant. Different types of bones (camel, cow and sheep) were pre-prepared by washing with distilled water and ignition at different heating temperatures from 600°C to 950°C in a muffle furnace for 2 h. Grind and washed with distilled water and finally dried at 60°C in drying oven. Nile Rose plant was treated by washed, cut and drying at 60°C in drying oven for 2 days.
Composition of natural sorbents
The stoichiometry of the constituents of the natural sorbents was determined using Philips Sequential X-ray spectrometer-2400. The solid samples were ground to very fine powders and then mixed with polyvinyl methacrylate as a binder to facilitate the pressing process. The mixture was pressed in a sample holder of 40 mm diameter aluminum cups and pressed on pressing machine at 20 psi to produce a sample with the diameter of 40 mm and 50 mm thickness. The concentrations of the main constituents of the natural sorbents were measured according to Super-Q quantitative application program.
Sorption studies
Time of equilibrium: Equal amounts of the sorbents were mixed with a stirred metal cations solution containing 50 mg/l for Cu2+, Mn2+, Ni2+ and Pb2+ ions at 25 ± 1°C for different intervals time between 5 min and 24 h. At definite intervals time, the solid was removed by filtration and the cation contents in the filtrate was determined by atomic absorption spectrometer.
Effect of batch factor: Equal amounts of the sorbents were mixed with different volumes of Cu2+, Mn2+, Ni2+ and Pb2+ ions with constant metal cations concentration (50 mg/l) for 3 h at 25 ± 1°C. After 3 h the solid was removed by filtration and the cation contents in the filtrate was determined by atomic absorption spectrometer.
Batch adsorption studies at various pH: The effect of pH of the medium on the distribution coefficients (Kd) of Cu2+, Mn2+, Ni2+ and Pb2+ ions on the natural sorbents were studied by addition of 0.1 g of the natural sorbents to 10 ml of 50 mg/l. The pH of the solution was adjusted to be in the range 1.0 to 5.0 for Cu2+, Ni2+, Pb2+ ions except for Mn2+ ion, precipitation is occurred at pH above 1.5. The exchange system was shaken in a shaker thermostat at 25 ± 1°C for 3 h (time to attain equilibrium) and the metal cation concentrations of Ni2+, Mn2+, Pb2+ and Cu2+ ions at the end of the study period were determined by atomic absorption spectrometer. The initial and final pH of solution was determined. Then the distribution coefficients were calculated by the following equation:
Kd=(A-Ao)/A .V/mml/g (1)
Where, Ao and A are the initial and final concentrations of metal cations in solution, respectively, V is the solution volume and m is the weight of the natural sorbents.
Absorption capacity measurements
Absorption capacity of natural sorbents for some heavy metals such as Cu2+, Mn2+, Ni2+ and Pb2+ ions (50 mg/l) was carried out using a batch technique with batch factor V/m equal to 100 ml/g and 200 ml/g for animal bones and Nile Rose plant, respectively. The experiments were done in a shaker water thermostat adjusted at 25 ± 1°C. After 3 h. time sufficient to attain equilibrium; the liquid phase was separated by centrifugation and analyzed by atomic absorption spectrometer for determination of the residue of metal ion concentration. The capacity in mmol/g was calculated using the following formula:
Capacity=(% Uptake/100) . Co . V/mmg/g (2)
where Co is the initial concentration of the ions in solution (mg/l), V is the solution volume (l) and m is the sorbent mass (mg).
Sorption isotherm
Sorption isotherms of Cu2+, Mn2+, Ni2+ and Pb2+ ions were determined over the entire metal ion concentration range 10-4 to 5 × 10-2 M at constant v/m ratio of 100 ml/g. After 3 h (time sufficient to attain equilibrium) the solution was separated and analyzed or determination of the residue of metal ion concentration. The equilibrium concentration (Ceq) and the amountsorbed (W) were calculated using the following relations:
W/m=Co x (uptake %/100) × V/m mmol/g (3)
Ceq=Co x [1-(uptake/100)] (4)
Where, W is the amount sorbed of metal ion, Co is the initial concentration of metal ion (mol/g), V is the solution volume (ml), m is the weight of the resin (g) and Ceq is equilibrium concentration. Plot of Ceq against C/W and Ceq against log C/W were performed to obtain the required isotherm.
Column technique
Chromatographic column with bed height 5 cm and internal diameter 1.2 cm was prepared by packing 0.5 g of natural sorbents. The simulated waste water with metal ion concentrations 50 mg/l was allowed to percolate through the natural sorbents at a flow rate of 1 ml/min Equal fractions of the effluent was collected and analyzed for the determination of the metal ion concentration in the effluent. Plot of C/Co against volume of effluent the breakthrough capacity (BTC) can be calculated using the following relation:
BTC=(V50%xCo)/mmg/g (5)
Where, V50% is the average breakthrough value representing at C/Co=50% in (ml), Co is the initial concentration of metal ion in the effluent (mg/l) and m is the weight of the natural sorbent (g).
Results, Discussion and Conclusion
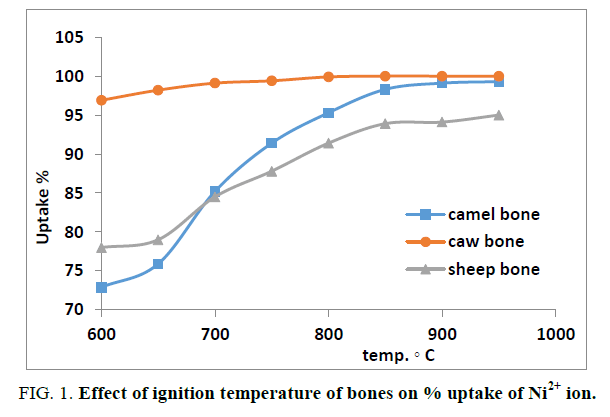
The scope of this work is the attempt to find a suitable natural sorbent for the removal of some hazardous heavy metals such as Ni2+, Mn2+, Cu2+ and Pb2+ produced from chemical industry factories. In this work we used different types of bones such as Camel, Cow and Sheep Bones and some plants such as Nile Rose plant. The elemental analysis of natural sorbents was conducted using X-ray fluorescence technique. The samples were ground to very fine powder and pressed in sample holder with 40 mm diameter and 5 mm thickness. The measurements were carried out according to Semi-Q application program. The summation concentration of the constituents obtained for Camel, Cow, Sheep and Nile Rose plant were 5.1%, 5.1%, 5.0% and 9.8%, respectively and all these data were normalized to 100.00% and tabulated in Table 1. From the data obtained we found that the concentration of calcium and phosphor ions was high in Camel bone compared to Cow and Sheep bones and show very low concentration in Nile Rose plant. On the other hand, silicone and aluminum concentration ions were lower in Camel bone compared to Cow and Sheep bones and show high concentration in Nile Rose plant. An ignition effect of the animal bones on the % uptake of cation was investigated to find the suitable heat treatment of bones. As sake of brevity, the effect of ignition on the % uptake of Ni2+ was conducted as shown in Figure 1. The data indicated that the % uptake of Ni2+ ion was increased with increasing an ignition temperature from the range 600°C to 950°C. For all further experiments bone was treated at 950°C.
| Analyte | Concentration, % | |||
|---|---|---|---|---|
| Camel bone | Cow bone | Sheep bone | Nile Rose plant | |
| Na | 9.86 | 11.37 | 17.01 | 14.80 |
| Mg | 2.39 | 2.07 | 5.51 | 9.34 |
| Al | 6.93 | 9.53 | 14.08 | 23.19 |
| Si | 15.10 | 22.28 | 28.48 | 29.88 |
| P | 13.67 | 8.88 | 6.90 | 2.36 |
| S | 3.38 | 2.35 | 4.88 | 3.76 |
| Cl | 6.64 | 3.91 | 9.27 | 10.02 |
| K | 1.14 | 1.26 | 1.55 | 3.46 |
| Ca | 28.71 | 36.24 | 11.18 | 2.37 |
| Fe | 0.89 | 0.71 | 0.48 | 0.49 |
| Ni | 0.31 | B.D.L. | B.D.L. | B.D.L. |
| Zn | B.D.L. | B.D.L. | B.D.L. | 0.15 |
| Pb | B.D.L. | 0.65 | B.D.L. | B.D.L. |
| Sr | 0.98 | 0.74 | 0.67 | 0.16 |
Data Normalized are 100%, B.D.L.: Below Detection Limit
Table 1: Phytochemical constituents of the chloroform extract of Annona muricata leaves.
The preliminary studies for the sorption of Cu2+, Mn2+, Ni2+ and Pb2+ ions on Camel, Cow and Sheep bones and Nile Rose plant were studied as shown in Figure 2 and indicated that, the equilibrium time was attained within 3 h. in a shaker thermostat adjusted at 25 °C ± 1°C. This time was taken in all further sorption experiments.
Figure 2: Effect of contact time on the % uptake of Ni2+, Mn2+, Pb2+ and Cu2+ ions on Camel, Cow, Sheep bones and Nile Rose plant at 25 ± 1°C.
The effect of the solution ion volume to the weight of the natural sorbents ration (batch factor) was studied to investigate the suitable ratio for maximum uptake of ions on Camel, Cow and Sheep bones and Nile Rose plant. In this study the effect of batch factor on the % uptake was conducted for Mn2+ ion as an example as a sake of brevity. The data in indicated that the batch factor has inversely effect for the uptake of Mn2+ ion on Camel, Cow and Sheep bones and proportionally effect on % uptake of Mn2+ ion on Nile Rose plant. The proportionally behavior of batch factor with the % uptake of Mn2+ ion on Nile Rose plant may be attributed to the very low density of Nile Rose plant and high surface area that leads to high contact of an exposed surface area of the sorbents with the solution may be due to an increasing ion the uptake of ions.
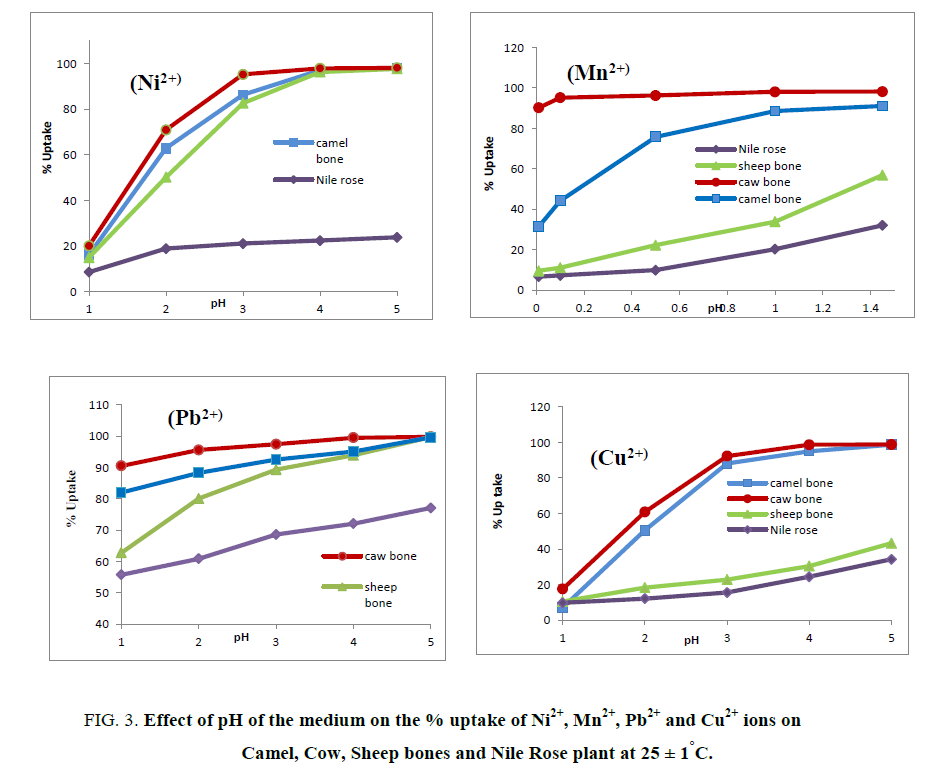
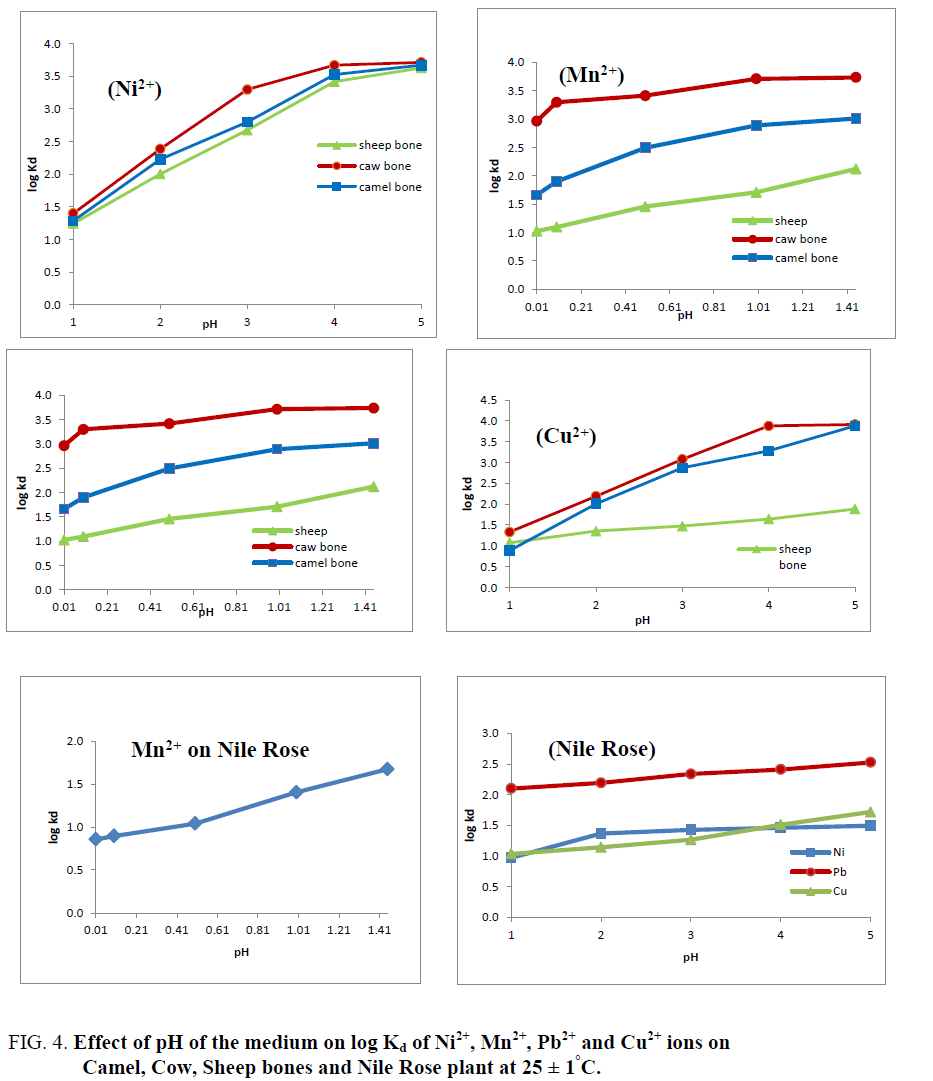
Effect of the pH of the medium on the % uptake and distribution coefficients of Cu2+, Mn2+, Ni2+ and Pb2+ ions on Camel, Cow and Sheep bones and Nile Rose plant were investigated as shown in Figure 3 and 4. As shown in Figure 3 it’s clear that as the pH of the medium has directly proportional with the % uptake of Cu2+, Mn2+, Ni2+ and Pb2+ ions on Camel, Cow and Sheep bones and Nile Rose plant. This behavior may be due to the cationic exchange properties of the natural sorbents used with the cations. The Kd values of Cu2+, Mn2+, Ni2+ and Pb2+ ions on Camel, Cow and Sheep bones and Nile Rose plant as a function of pH of solution at constant concentration of the cations in solution are represented in Figure 4. This figure showed that, the Kd values of Cu2+, Mn2+, Ni2+ and Pb2+ ions are increased with increasing the pH of the solution from 1.0 to 5.0. This behavior may be attributed to the high concentration of the H+ ion in the feed solution at lower pH so the retention of studied elements Cu2+, Mn2+, Ni2+ and Pb2+ ions from solution are decreased, as the pH increased the H+ ion in the feed solution are decreased and the chance of the exchange are increased leading to an increasing in the Kd values of Cu2+, Mn2+, Ni2+ and Pb2+ ions on animal bones and Nile Rose plant to different extent.
Figure 3: Effect of pH of the medium on the % uptake of Ni2+, Mn2+, Pb2+ and Cu2+ ions on Camel, Cow, Sheep bones and Nile Rose plant at 25 ± 1°C.
Figure 4: Effect of pH of the medium on log Kd of Ni2+, Mn2+, Pb2+ and Cu2+ ions on Camel, Cow, Sheep bones and Nile Rose plant at 25 ± 1°C.
Figure 4 also generally showed that the selectivity order of the studied cations on the natural sorbents used have the order:
Pb2+>Ni2+>Mn2+>Cu2+. The nonlinear relation between log Kd and pH was observed as predicted from the following:
When the simple ion exchange proceeds by the following reaction:
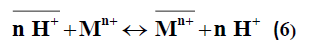
in sufficiently diluted solution, where activity coefficient may be neglected, the selectivity coefficient can be defined by the following equation [11]:
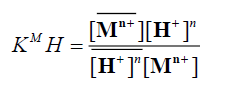 (7)
(7)
where 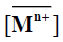 and
and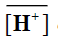 denote to the concentrations of Mn+ and H+ ions in the exchanger, respectively and [Mn+] and [H+] are their concentrations in solution. Since the Kd values is the ratio between the metal ion concentration in the exchanger and in the solution, then,
denote to the concentrations of Mn+ and H+ ions in the exchanger, respectively and [Mn+] and [H+] are their concentrations in solution. Since the Kd values is the ratio between the metal ion concentration in the exchanger and in the solution, then,
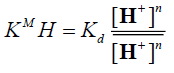 (8)
(8)
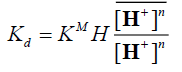 (9)
(9)
By taking the logarithm of the two sides,
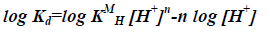 (10)
(10)
When 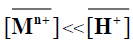 and [Mn+]<<[H+], the magnitude KM H [H+]n is considered constant, thus equation (10) can be reduced
and [Mn+]<<[H+], the magnitude KM H [H+]n is considered constant, thus equation (10) can be reduced
log Kd=C +npH (11)
This implies that a plot of log Kd versus pH should be linear with a slope (n). Figure 4 shows the dependency of Kd values of Cu2+, Mn2+, Ni2+ and Pb2+ ions on the pH of the solution for Camel, Cow and Sheep bones and Nile Rose plant with nonlinear relation, which improving the non-ideality of the exchange reaction. The non-ideality results may be due to the presence of other mechanism beside the ion exchange mechanism such as adsorption and the formation of covalent bonds between the metal ions and the natural sorbents that could be related to the ionic potential of the cations. From Figure 4 we found that animal bones have high selectivity for Cu2+, Mn2+, Ni2+ and Pb2+ ions compared to Nile Rose plant with the selectivity sequence; Cow bone>Camel bone>Sheep bone>Nile Rose Plant.
The results of adsorption capacities of Cu2+, Mn2+, Ni2+ and Pb2+ ions on Camel, Cow and Sheep bones and Nile Rose plant are shown in Table 2. The data shown in Table 2 showed that the efficiency of animal bones for Cu2+, Mn2+, Ni2+ and Pb2+ are higher than these obtained for Nile Rose plant which may be due to the higher calcium content of Bones than Nile Rose plant (Table 1) which may be act as an exchangeable active site. Table 2 also showed that, the selectivity sequence for the studied cations are Pb2+>Mn2+>Ni2+>Cu2+ for Cow and Sheep bones and Mn2+>Pb2+>Ni2+>Cu2+ for Camel and Nile Rose plant. This selectivity sequence agrees with the atomic radius of these cations that means the adsorption process were carried out in dehydrated states [10]. Similar results were reported by Abou-Mesalam et al. for the adsorption of Ni2+, Zn2+, Cd2+ and Cu2+ ions on SiTi and SiSb as inorganic ion exchange materials [11].
| Ions | Capacity, mg/g | |||
|---|---|---|---|---|
| Cow bone | Camel bone | Sheep bone | Nile Rose plant | |
| Mn2+ | 19.9 | 16.33 | 19.3 | 9.14 |
| Cu2+ | 1.86 | 1.23 | 1.86 | 0.40 |
| Ni2+ | 9.74 | 9.70 | 9.80 | 4.80 |
| Pb2+ | 20.74 | 14.1 | 25.21 | 4.69 |
Table 2: Capacities of Camel, Cow and Sheep bones and Nile Rose plant for Cu2+, Mn2+, Ni2+ and Pb2+ ions at 25 ± 1°C.
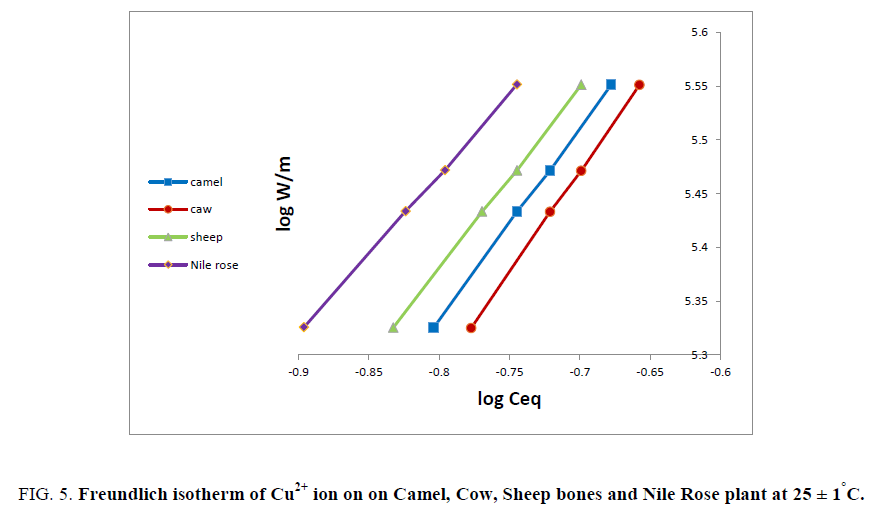
The nature of adsorption processes for Mn2+, Pb2+, Ni2+ and Cu2+ ions on different sorbents used were investigated by gradual increase of the sorbate concentration and measuring the amount sorbed at each equilibrium concentration. The Freundlich isotherm most widely used mathematical model, given an empirical expression encompassing the surface heterogeneity and exponential distribution of active sites and their energies was tested in the following form:
log W/m=K + K' log Ceq. (12)
Where, W is the amount uptake,
Ceq. is equilibrium concentration,
K and K' are the Freundlich constants measure the adsorption intensity and adsorption capacity of the sorbent, respectively and computed from the slope and intercept of the linear relationship.
As sake of brevity, Cu2+ ion was reported as an example for the plots of log W/m against log Ceq. Figure 5 shows linear relationships were obtained for the sorption of Cu2+ ion on all different natural sorbents used. The data in Figure 5 shows the applicability of Freundlich isotherm for Cu2+ ion and is physically sorbed on animal bones and Nile Rose plant sorbents. The values of adsorption capacity (K) and adsorption intensity (K') for Cu2+ ion on bones and Nile Rose plant were computed from the linear relationships in Figure 5 and the numerical values of (K'<1) suggest the surface of the sorbent of heterogeneous type [12]. Also the numerical value of (K') is only reduced at lower equilibrium concentrations. Freundlich sorption isotherm does not predict any saturation of the solid surface thus envisages infinite surface coverage mathematically. Similar results were also reported for the adsorption of Zn2+, Cu2+, Cd2+ and Ni2+ ions on poly acrylamide acrylic acid impregnated with silico-titanate ion exchanger [13] and UO22+ and Th4+ ions on titanium antimonate [14].
Figure 5: Freundlich isotherm of Cu2+ ion on on Camel, Cow, Sheep bones and Nile Rose plant at 25 ± 1°C.
Solid bed columns were used to explore suitable conditions for quantitative loading and sorption of some toxic elements (Mn2+, Pb2+, Ni2+ and Cu2+) from industrial waste. In agreement with the batch distribution data, the adsorption of the respective metal ions on bone and Nile Rose column beds was carried out at pH 3.0 for Pb2+, Ni2+ and Cu2+ ions and pH 1.4 for Mn2+ ion. In addition, the adsorption of different cations from waste solution is markedly improved when bones are used rather than Nile Rose plant. Figure 6 show the breakthrough curves for each of Mn2+, Pb2+, Ni2+ and Cu2+ ions on Cow, Camel and Sheep bones and Nile Rose plant columns. From these figures breakthrough capacity of these cations on different bones and Nile Rose plant were calculated and the selectivity order on the different bones and Nile Rose plant was found to; Pb2+ (13.94 mg/m)>Mn2+ (8.77 mg/g)>Ni2+ (11.18 mg/g)>Cu2+ (2.72 mg/g) for Cow bone, Mn2+ (21.93 mg/g)>Ni2+ (13.97 mg/g)>Pb2+ (5.58 mg/g)>Cu2+ (1.36 mg/g) for Camel bone; Mn2+ (39.45 mg/g)>Ni2+ (16.77 mg/g)>Pb2+ (13.94 mg/g)>Cu2+ (2.72 mg/g) for Sheep bone; and Mn2+ (48.23 mg/g)>Pb2+ (25.09 mg/g)>Ni2+ (13.97 mg/g)>Cu2+ (3.06 mg/g). The data in Figure 6 was also indicated that these cations show breakthrough tailing on Nile Rose plant column due to slow dynamic equilibrium. When the ionic charge of the metal ions in the feed solution are considered, the uptake for any of them per gram of the sorbing was found to be in the range 2.72 mg/g to 13.94 mg/g of Cow bone, 1.36 mg/g to 21.93 mg/g of Camel bone, 2.72 mg/g to 39.45 mg/g of sheep bone and finally, 3.06 mg/g to 48.23 mg/g of Nile Rose plant.
References
- El-Naggar IM, Abou-Mesalam MM, El-Shorbagy MM, et al. First Conf. on Trends in Waste Recycling, Helwan University, Cairo Egypt. 2002.
- Kladov A. Radiochemical Conference. 19-24 April 1998, Marianske Lazne Jachymov, Chech Republic, Booklet of Abstracts.
- El-Naggar IM, Abou-Mesalam MM. Novel inorganic ion exchange materials based on silicates; synthesis, structure and analytical applications of magneso-silicate and magnesium alumino-silicate sorbents. Journal of Hazardous Materials. 2007;149(3):686-92.
- Siyam T, Youssef HA, El-Naggar IM. Adsorption studies of copper sulfate on hydrogels of poly (amido-amines). Journal of Macromolecular Science. Part A: Pure and Applied Chemistry. 1997;34(11):2379-88.
- Kanno S, Hosoi M, Ogata T, et al. Polymerization of Phenylmaleimide Initiated by 9-Borabicyclo [3.3. 1] nonane. Polymer International. 1997;42(3):321-7.
- Lin W, Hsieh YL. Ionic absorption of polypropylene functionalized by surface grafting and reactions. Journal of Polymer Science Part A: Polymer Chemistry. 1997;35(4):631-42.
- El-Rehim HA, Hegazy EA, Ali AE. Selective removal of some heavy metal ions from aqueous solution using treated polyethylene-g-styrene/maleic anhydride membranes. Reactive and Functional Polymers. 2000;43(1):105-16.
- Siyam T. Possible mechanisms for interaction of polyelectrolytes with ions in aqueous solution. Journal of Macromolecular Science, Part A. 1995;32(sup1):801-11.
- Shady SA. Chemical studies for the removal of some heavy metals from toxic waste solutions using organic and inorganic sorbents. Ph.D. Thesis, Chemistry Department, Faculty of Science, Ain Shams University. 2001.
- Greig JA. Ion exchange developments and applications: Proceedings of IEX'96. Royal Society of Chemistry; 1996.
- Abou-Mesalam M. Retention behavior of nickel, copper, cadmium and zinc ions from aqueous solutions on silico-titanate and silico-antimonate used as inorganic ion exchange materials. Journal of Radioanalytical and Nuclear Chemistry. 2002;252(3):579-83.
- Mishra SP, Srinivasu N. Ion exchangers in radioactive waste management. Part VI: radiotracer studies on adsorption of barium ions on potassium titanate. Radiochimica Acta. 1993;61(1):47-52.
- Abou-Mesalam MM, Mostafa MM, Abdel A, et al. Chemical studies on the retention of some heavy metals from simulated waste water using polymeric species impregnated inorganic ion exchanger. Arab Journal of Nuclear Sciences and Applications. 2005;38(3):53-62.
- Ibrahim GM. M. Sc. Thesis, Faculty of Science, Cairo University, Cairo, Egypt. 1997.