Original Article
, Volume: 12( 12)Bio-Synthesis of Iron Nanoparticles using the Brown Seaweed, Dictyota dicotoma
- *Correspondence:
- Chandran M , Department of Biotechnology, Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College, Chennai, India,
Tel: 073587 01998; E-mail: biochandran1976@gmail.com
Received: November 07, 2016; Accepted: November 20, 2016; Published: November 26, 2016
Citation: Chandran M, Yuvaraj D, Christudhas L, et al. Bio Synthesis of Iron Nanoparticles using the brown Seaweed, Dictyota dicotoma. Biotechnol Ind J. 2016;12(12):112.
Abstract
Biosynthesis of metal nanoparticles using plants is currently focal point in nanomaterial studies. Green synthesis of metal nanoparticles has an important role to play in the future of medicine. This research paper focuses on the utilization of seaweed for the biosynthesis of iron nanoparticles in ambient conditions without any additives. The brown seaweed Dictyota dicotoma, was collected from the Muttom region in Kanyakumari district and utilized for the synthesis of iron nanoparticles. The NPs were characterized by UV-Visible spectrophotometer and FTIR. The nanoparticles produced were in the size range between 40 nm-50nm which was confirmed from the SEM analysis. The magnetic properties of the NPs were studied. The antimicrobial potential of the synthesized NPs were also evaluated. Nanoparticles coupled with antibiotics showed a greater inhibitory effect than the antibiotic and the synthesized nanoparticle’s individual performance.
Keywords
Seaweed; Nano particles; FTIR; SEM; UV-Visible
Introduction
Nano science and technology is developing into one of the most important areas of research in the recent years. Nanotechnology implies the creation of nanoparticles in size, shape, chemical compositions and controlled diversity and their potential uses for human benefits [1]. Nanoparticles are produced by chemical and physical methods are expensive, non-reliable and potentially dangerous to the environment. Alternatively, nanoparticles produced by biological methods seem to gain interest as they are supposed to be inexpensive and eco-friendly. Biosynthesis of nanoparticles is a kind of bottom up approach and mainly by oxidation and reduction technique [2]. Nanotechnology has a wide range of applications mainly in the field of biomedical applications, drug delivery system, ultra-sensitive disease detection, etc. [3,4].
Biosynthesis of metal nanoparticles using the plants is currently under development. Green synthesis of metal nanoparticles has an important role to play in the future of medicine [5]. This research paper focuses on the utilization of seaweed for the biosynthesis of iron nanoparticles in ambient conditions without any additive from any aggregation.
Materials and Methods
Ferric chloride (2%), ferrous sulphate (1%) and sodium hydroxide were used for the preparation of samples. The glass wares used in this experimental work were acid washed. Ultrapure water was used for all dilution and sample preparation.
Seaweeds collection
Seaweeds were collected from Muttom, Kanyakumari district, Tamil Nadu coastal region and identified as Dictyota dichotoma (Figure 1).
Preparation aqueous extract
The collected seaweed samples were washed in seawater and then fresh water to remove epiphytes and other contamination. Then the seaweeds ware washed thoroughly thrice with distilled water and was shade dried for 10 days. Fine powder of the seaweeds was obtained by using kitchen blender. The seaweed powder was sterilized at 120°C for 15 min.
About 20 g of seaweed powder was taken and mixed with 200 ml of distilled water in an Erlenmeyer flask and kept in heating mantle at 60°C for 20 min. The extracts were filtered with Whatman No.1 filter paper and stored in refrigerator at 4°C for further studies (Figure 2).
Green synthesis of iron nanoparticles
Iron nanoparticles were prepared by adding ferric chloride (2%), ferrous sulphate (1%) solution, to the extracts (10 ml) and precipitated with 2 ml sodium hydroxide (0.1 M). The pH of the solution was controlled by means of a buffer solution, fixing the values between 7 and 10 while stirring vigorously. An orange precipitate immediately formed. The prepared emulsion was placed on a magnetic stirrer and stirred for 1 h at room temperature. This procedure was applied to four different seaweed extracts. The black color precipitate was formed. The pH was maintained between 7 and 10 which was adjusted using 0.1 M Hcl or 0.1 M NaOH If 1 ml of 1 M HCl is added to 1 L of pure water, the pH will change from 7 to 3 (a change of 4 pH units); duration of reaction time was maintained from 1 h to 72 h. The color change indicates the formation of iron Nano particles (Figure 3).
The black precipitate, thus obtained was washed thrice with deionized water and twice with ethanol. The black colored nanoparticles were separated by magnetic decantation to form magnetite. The magnetites formed were washed 3 times with deionized water and 2 times with ethanol, and dried at room temperature (Figure 4).
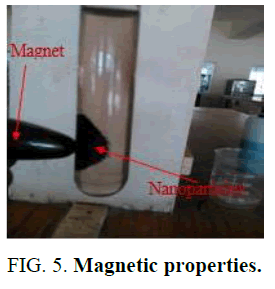
The magnetic property of the Nano particle was checked by using the external magnet (Figure 5).
Characterization methods
In order to investigate various properties of the prepared sample, it has to goes under a number of characterization techniques. The results give the information about the different optical and structural properties of sample. The synthesized nanoparticles were analyzed using ultra violet–visible spectroscopy (UV-Vis) (Perkin-Elmer Lambda 2 Spectrophotometer) in 300 nm to 700 nm wave length range.
As we know iron oxide has wide range of application in the field of optoelectronics devices on putting the sample to following characterization techniques gives information related to optical properties [6-10].
• UV-Visible spectroscopy (UV)
• Fourier transform infrared spectroscopy (FTIR)
In order to get exact information about the crystal structure, surface morphology, particle size etc. the following characterization techniques are applicable
• SEM (Scanning Electron Microscope)
Antibacterial activity of iron nanoparticles
Antibacterial activity of iron oxide nanoparticles was analyzed using well diffusion method against selected bacterial species. The inhibitory activity of the Iron nanoparticles was evaluated against pathogenic bacteria and their potency was assessed qualitatively by the presence of inhibition zones. Different classes of bacteria exhibit different susceptibilities to nanoparticles. Iron nanoparticles combined with antibiotic drugs showed excellent antibacterial activity against the bacterial pathogens.
Results and Discussion
UV-Spectroscopy
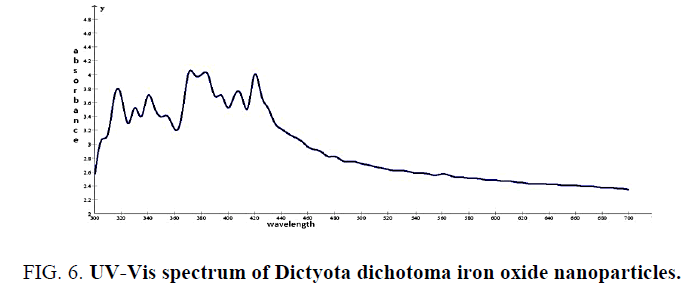
The biologically synthesized iron nanoparticles were analyzed using ultra violet-visible spectroscopy (UV vis) in 300 nm to 700 nm wave length range. The spectra clearly shows maximum absorption peaks which indicates the formation of increased number of iron nanoparticles in the solution. Quite interestingly, the solution was extremely stable even after 3 months of reaction, with no evidence of aggregation of particles.
The absorption peaks at wavelengths of 320 nm, 370 nm and 420 nm indicate the formation of iron nanoparticles (Figure 6 and 7).
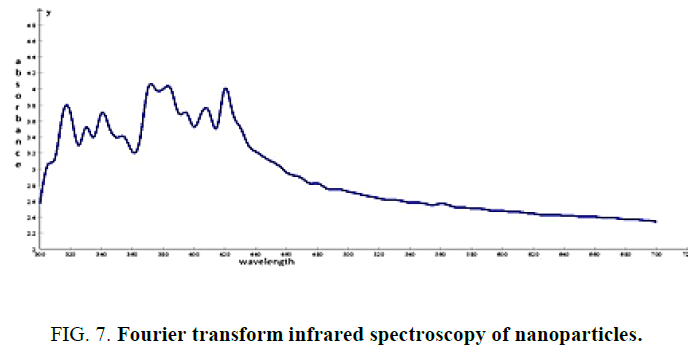
Figure 7: Fourier transform infrared spectroscopy of nanoparticles.
Fourier transform infrared spectroscopy
FTIR is an analytical instrument plays a major role in determining the functional groups of many organic and inorganic compounds. The wavelength of light absorbed is characteristic of the chemical bond as can be seen in the annotated spectrum. By interpreting the infrared absorption spectrum, the chemical bonds in a molecule can be determined [11]. The peak at 3419 and 2906 cm-1 are corresponded to Hydroxyl and CH stretching frequency respectively. The peak at 1629 cm-1 to assign C=C [12], 2653 (carboxylic acid -O-H stretching), 1378 (alkane CH3 bending), 1105 (C-O) stretching [13]. Some of these functional groups satisfies the phytochemical analysis of Dictyota dichotoma [14].
SEM analysis of iron nanoparticles
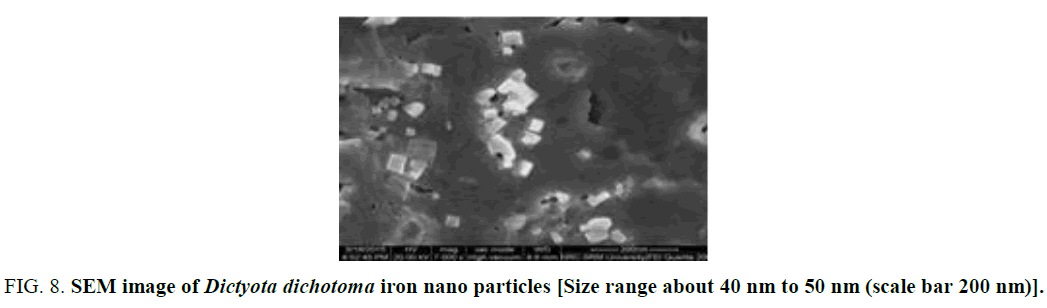
The electron microscopy can be employed to characterization the size, shape and morphologies of formed iron Nano particles. The SEM images for samples are shown in figure respectively (Figure 8).
Figure 8: SEM image of Dictyota dichotoma iron nano particles [Size range about 40 nm to 50 nm (scale bar 200 nm)].
Evaluation of antibacterial activity
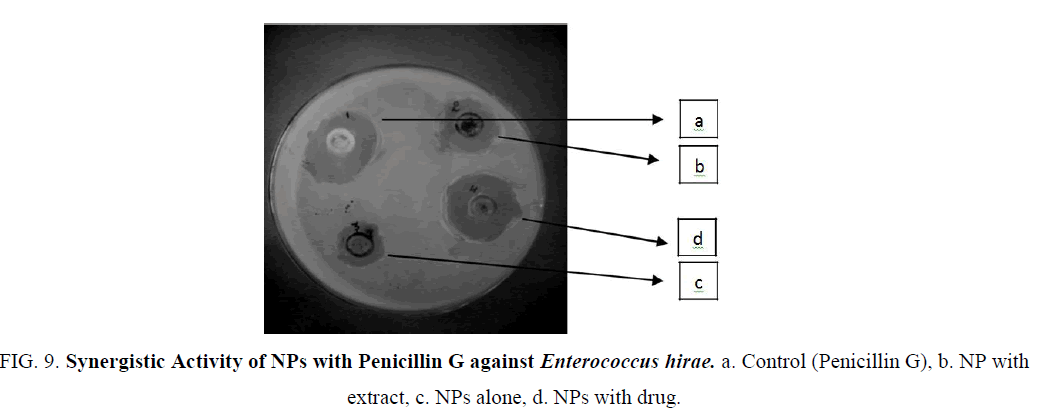
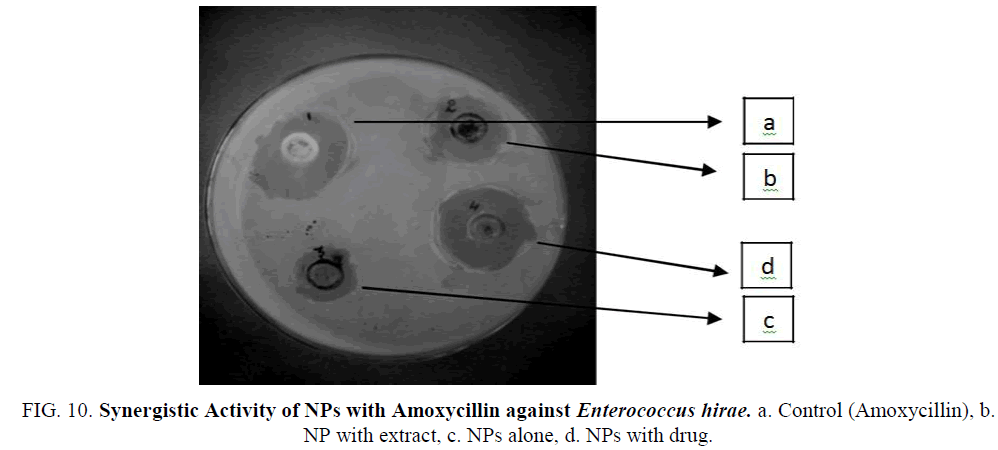
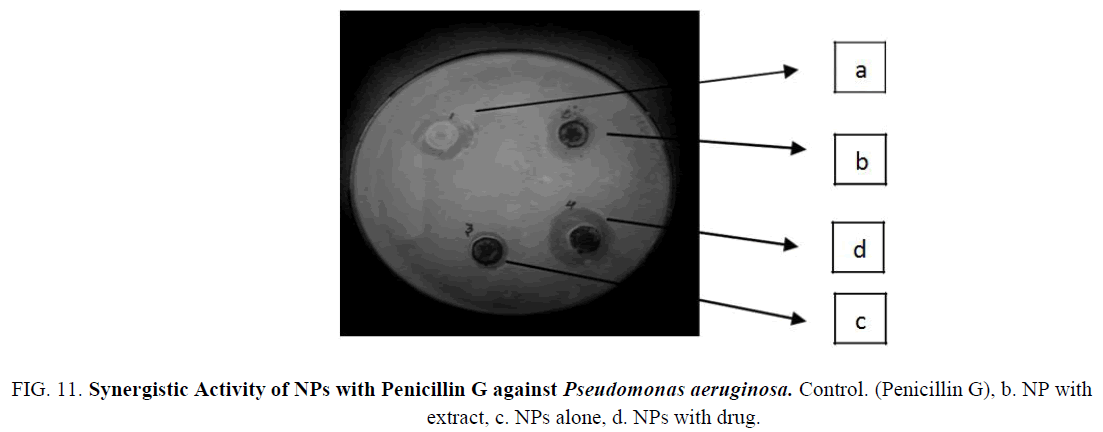
The Iron nanoparticles synthesized was tested for antibacterial activity by agar well-diffusion method against Pseudomonas aeruginosa (gram negative), Escherichia coli (gram negative), Enterococcus (gram positive). The pure bacterial culture was subcultured on nutrient agar. Wells of 10 mm diameter were made on nutrient agar plates using gel puncture. The strain was swabbed uniformly onto the individual plates using sterile cotton swabs. Using a micropipette, 50 μl of nanoparticle solution, antibiotic (Penicillin G and Amoxycillin), and their combination and was poured onto each well on the plates. After incubation at 37°C for 24 h, the different level of zone of inhibition of bacteria was measured (Table 1) (Figure 9-14).
| E. hirae | Penicillin G | 6 mm | 1 mm | 3 mm | 7 mm |
| E. hirae | Amoxycillin | 8 mm | 2 mm | 4 mm | 8 mm |
| P. aeruginosa | Penicillin G | 3 mm | 1 mm | 4 mm | 6 mm |
| P. aeruginosa | Amoxycillin | 2.3 mm | 1.8 mm | 2.4 mm | 5 mm |
| E. coli | Penicillin G | 5 mm | - | 3 mm | 7 mm |
| E. coli | Amoxycillin | 5 mm | - | 4 mm | 8 mm |
Table 1: Minimum inhibitory zones of NPs and NP drug conjugation.
Figure 9: Synergistic Activity of NPs with Penicillin G against Enterococcus hirae. a. Control (Penicillin G), b. NP with extract, c. NPs alone, d. NPs with drug.
Figure 10: Synergistic Activity of NPs with Amoxycillin against Enterococcus hirae. a. Control (Amoxycillin), b. NP with extract, c. NPs alone, d. NPs with drug.
Figure 11: Synergistic Activity of NPs with Penicillin G against Pseudomonas aeruginosa. Control. (Penicillin G), b. NP with extract, c. NPs alone, d. NPs with drug.
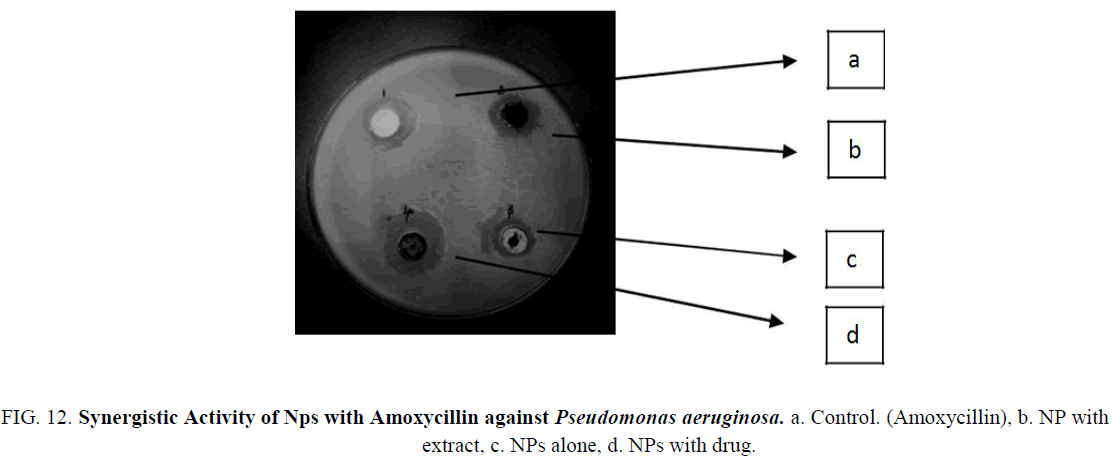
Figure 12: Synergistic Activity of Nps with Amoxycillin against Pseudomonas aeruginosa. a. Control. (Amoxycillin), b. NP with extract, c. NPs alone, d. NPs with drug.
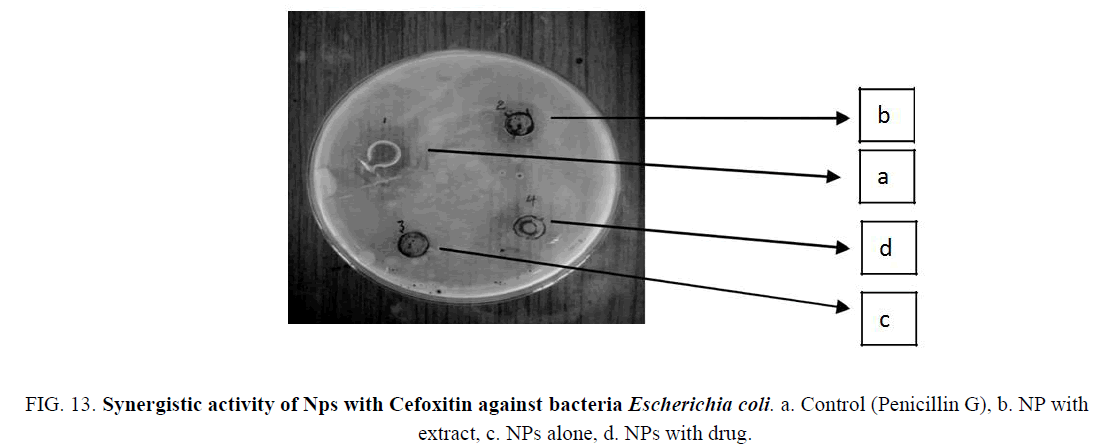
Figure 13: Synergistic activity of Nps with Cefoxitin against bacteria Escherichia coli. a. Control (Penicillin G), b. NP with extract, c. NPs alone, d. NPs with drug.
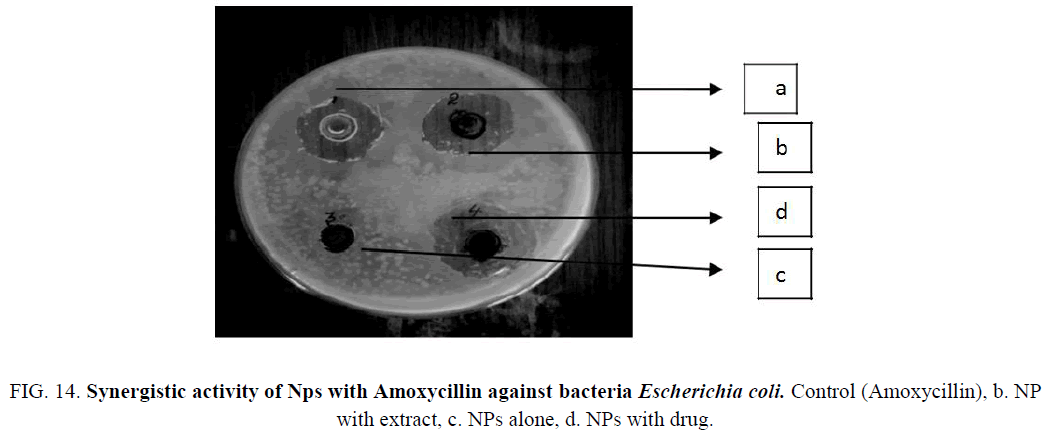
Figure 14: Synergistic activity of Nps with Amoxycillin against bacteria Escherichia coli. Control (Amoxycillin), b. NP with extract, c. NPs alone, d. NPs with drug.
Conclusion
Biosynthesis of iron nanoparticles using Dictyota dicotoma was carried out and the results are interpreted using different analytical instruments like UV-Spectrophotometer which indicate the formation of iron nanoparticles at 320 nm, 370 nm and 420 nm, followed by FTIR that was used in identifying the functional groups present in synthesized nanoparticles and SEM images showed the relative size of the synthesized nanoparticles in the range of 40 nm to 50 nm. Further studies showcased the antimicrobial property of the synthesized nano particle solution, antibiotic (Penicillin G, Amoxicillin) and their combination against selected species of bacteria, Pseudomonas aeruginosa (gram negative), Escherichia coli (gram negative), Enterococcus hirae (gram positive) by agar well diffusion method. The zone of inhibition is comparatively high in nanoparticle conjugate with antibiotics than the individual performances. The results were so encouraging that future research can be done with disease specific drug conjugates.
References
- Giridharan T, Chandran Masi S, Sindhu, et al. Studies on green synthesis, Characterization and Anti-proliferative Potential of Silver Nano Particle using Dodonaea viscosa and Capparis deciduas. Biosci Biotechnol Res Asia. 2014;11(2):665-73.
- Curtis ASG, Wilkinson C. Nanotechniques and approaches in biotechnology. Trends Biotech. 2001;19:97-101.
- Wilkinson JM. Nanotechnology applications in medicine. Med Device Technol. 2003;14(5):29-31.
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 55:329-47.
- Giridharan T, Chandran M, Sindhu S, et al. Comparative studies on green synthesis and therapeutic applications of silver nano particles using Flacourtia Sepiaria and Rhinacanthus Nasutus. Int J Pharm Bio Sci. 2014;5 (4):560-9.
- Tapan K, Jain Marco A, Morales, et al. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2(3):194-205.
- Kaushik A, Raju K, Pratima R, et al. Iron oxide nanoparticles-chitosan composite based glucose biosensor. Biosens Bioelectron. 2008;24:676-83.
- Nidhin M, Indumathy R, Sreeram KJ, et al. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull Mater Sci. 2008;31(1):93-6.
- Ahmed S, Al-Kadya, Gabera M, et al. Structural and fluorescence quenching characterization of hematite nanoparticles. Spectrochimica Acta Part A. 2011;83:398-405.
- Wang L, Luo J, Mathew M, et al. Iron oxide-gold core-shell nanoparticles and thin film assembly. J Mater Chem. 2005;15:1821-32.
- Ashokkumar R, Ramaswamy M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian Medicinal plants. Int J Curr Microbiol App Sci. 2014;3(1):395-406.
- Starlin T, Arul Raj C, Ragavendran P, et al. Phytochemical screening, functional groups and element analysis of Tylophora Pauciflora wight and ARN. IRJP. 2012;3(6):180-3.
- Mahesha M, Poojary, Kanivebagilu A, et al. Extraction, characterization and biological studies of phytochemicals from Mammea suriga. 2015;5(3):182-9.
- Deyab MD, Elkatony T, Ward F. Qualitative and quantitative analysis of phytochemical studies on brown Seaweed, Dictyota dichotoma. IJEDR. 2016;4(2):674-8.