Review
, Volume: 13( 1)Bio-Pharmaceutical Manufacturing Large Scale Production Process: The Graphene Derivates Role and mRNA Vaccine
Mauro Luisetto1*, Tarro G2, Naseer Almukthar3, Nili B Ahmadabadi4, Ram Sahu5, Farhan Ahmad Khan6, Mashori Gulam Rasool7, Cabianca L8, Fiazza C9, Gadama G Prince10, Oleg Yurevich Latyshew11
1Department of Toxicology and Pharmacology, IMA academy, Natural science Branch, Pavia, Italy
2Department of Oncology, President of the T and L de Beaumont Bonelli Foundation for Cancer Research, University of Naples, Naples, Italy
3Department of Physiology, University of Babylon, Babylon, Iraq
4Department of Nanomedicine, The University of North Carolina, Chapel Hill, United States
5Department of Pharmaceutical Science, Institute of Pharmaceutical Science, Assam University, Silchar, Assam, India
6Department of Pharmacology, Institute of Pharmaceutical Science, Jawaharlal Nehru Medical College, AMU, Aligarh, India
7Department of Medical and Health Sciences for Woman, Institute of Pharmaceutical Science, Peoples University of Medical and Health Sciences for Women, Sindh, Pakistan
8Department of Pharmacology, Institute of Pharmaceutical Science, University of Pavia, Pavia, Italy
9Department of Science, Malawi Institute of Pharmaceutical Science, University of Science and Technology, Malawi, East Africa
10Department of Science, Institute of Pharmaceutical Science, Chair Cypress University, Malawi, East Africa
11Department of Science, Institute of Pharmaceutical Science, IMA Academy Marijnskaya President RU, Russia
- *Correspondence:
- Mauro Luisetto
Department of Toxicology and Pharmacology, University of Pavia, Pavia, Italy
Tel: 393402479620
E-mail: maurolu65@gmail.com
Received: September 12, 2022, Manuscript No. TSJCCPS-22-74370; Editor assigned: September 14, 2022, PreQC No. TSJCCPS-22-74370 (PQ); Reviewed: September 28, 2022, QC No. TSJCCPS-22-74370; Revised: January 16, 2023, Manuscript No. TSJCCPS-22-74370 (R); Published: January 23, 2023, DOI: 10.37532/2277-2871.23.13.002
Citation: Luisetto M, Tarro G, Almukthar N, et al. Bio-Pharmaceutical Manufacturing Large Scale Production Process: The Graphene Derivates Role and mRNA Vaccine. J Curr Chem Pharm Sci. 2023;13(1):002
Abstract
Aim of this work is to investigate the role played by graphene and its derivates in some relevant manufacturing process like purification and absorption. The chemical physical properties of this innovative product make possible to understand why they are used in many biomedical and other fields like biosensors, in water purifying, to remove heavy metals procedure, in diagnostic field, but also in pharmaceutical purifications, in extraction, purifying DNA, RNA and other biomolecule, carrier, adjuvant, antibacterial and other biological and industrial use. The large scale production is different from a laboratory scale but the same chemicophysical properties are used. The classic drug manufacturing production as well as biopharmaceuticals needs to verify the presence of Impurity in the raw material and in the finished drugs for regulatory need and for patient safety. For this reason it is interesting to verify the use in some large scale manufacturing procedure of innovative products like m RNA Vaccine. Because this products graphene oxide and other product related is recognized with toxicological properties it is strongly recommended by the authors if used in the manufacturing process to test in the final product the full absence of this molecule with written report inside the technical sheet approved.
Keywords
Graphene; Graphene oxide; Pharmaceutical purification; Process; Manufacturing; Biopharmaceutical; Raw materials; Toxicology; GMP; Impurity; Quality control; mRNA vaccine; COVID-19; Graphene magnetic beads; Regulatory process
Introduction
Biopharmaceuticals have revolutionized the field of medicine in the types of active ingredient molecules and treatable indications. Adoption of quality by design and Process Analytical Technology (PAT) frameworks has helped the biopharmaceutical field to realize consistent product quality, process intensification and real time control CQ. As part of the PAT strategy, Raman spectroscopy offers many benefits and is used successfully in bioprocessing from single cell analysis to cGMP process control. Since first introduced in 2011 for industrial bio processing applications, Raman has become a first choice PAT for monitoring and controlling upstream bio processes because it facilitates advanced process control and enables consistent process quality. This work will discuss new frontiers in extending these successes in upstream from scale down to commercial manufacturing. New reports concerning the use of Raman spectroscopy in the basic science of single cells and downstream process monitoring illustrate industrial recognition of Raman’s value throughout a bio pharmaceutical product’s lifecycle. Raman spectroscopy has been used since 2006 to provide analytical quality control of compounded formulations stored in vials or directly through polymeric infusion pumps in hospital settings [1].
Before to start this work it is interesting to observe the various research and applications of graphene derivates in field of chemistry, environment science, biotechnology and medicine. All these uses are related the peculiar chemicophysical properties of this molecule and this is obvious to all. But if it is clear that in last decades many technological application was introduced in materials and in procedures related it is relevant to observe also the profile of toxicity of this products. Useful materials yes but it must to be deeply verified what happen when used this technology. This work is not focused on clinical toxicology aspect but only on what happen during and after some industrial large scale manufacturing of bio-pharmaceuticals using graphene and its derivates. This products make possible to increase efficiency in production but are we sure that not become impurity or contaminants for the bio products produced using this kind of chemical substantial? And what it means when a great pharmaceutical industry related PEG2000-DMG control recipient used for a famous mRNA COVID-19 vaccine: In the EMA 2021 assessment report it was written, ‘‘The specification is currently not acceptable” and “The current reporting of impurities is not acceptable”.
Polyethylene Glycol (PEG) is widely utilized in drug delivery and nano technology due to its reported “stealth” properties and biocompatibility. It is generally thought that PEGylation allows particulate delivery systems and bio materials to evade the immune system and thereby prolong circulation lifetimes.
EMA has analyzed reports describing the analysis of several vials of COVID-19 vaccines suggesting the presence of graphene and concluded that the currently available data do not show presence of graphene in the vaccines concerned. The analysis by EMA’s working party for biological medicines included an input on the Raman spectroscopy from the European directorate for quality of medicines and the independent national testing laboratories responsible for the batch release (OMCLs).
Graphene Oxide (GO) is not used in the manufacture or formulation of any of the COVID-19 vaccines or other medicines, so it would not be present at manufacturing facilities and there is no obvious way that it could get into the vaccines. Quality control testing and quality assurance review, by the vaccine manufacturers and OMCLs responsible for batch release, confirm that each batch met all quality standards prior to the release. No product complaints have been received for the batches mentioned in the paper. The presence of graphene or graphene derivatives in the vaccines therefore is not plausible. The commission and EMA do not consider that any further actions are necessary at this stage.
An electron microscopy investigation method was applied to the study of vaccines, aimed at verifying the presence of solid contaminants by means of an environmental scanning electron microscope equipped with an X-ray microprobe. The results of this new research show the presence of micro and nano sized particulate matter composed of inorganic elements in vaccines samples which is not declared among the components and whose unduly presence is, for the time being, inexplicable. A considerable part of those particulate contaminants have already been verified in other matrices and reported in literature as non-bio degradable and non-bio compatible. 44 types of vaccines coming from 2 countries (Italy and France) were analyzed. Table reported groups them in terms of name, brand and purpose [2].
Literature Review
The analyses carried out show that in all samples checked vaccines contain non biocompatible and bio persistent foreign bodies which are not declared by the producers, against which the body reacts in any case. The investigations verified the physical chemical composition of the vaccines considered according to the inorganic component as declared by the producer. In detail, we verified the presence of saline and aluminum salts, but further presence of micro, sub micro and nanosized, inorganic, foreign bodies (ranging from 100 nm to about ten microns) was identified in all cases, whose presence was not declared in the leaflets delivered in the package of the product.
This new investigation represents a new quality control that can be adopted to assess the safety of a vaccine. Our hypothesis is that this contamination is unintentional, since it is probably due to polluted components or procedures of industrial processes (in e.g. filtrations) used to produce vaccines, not investigated and not detected by the producers. If our hypothesis is actually the case, a close inspection of the working places and the full knowledge of the whole procedure of vaccine preparation would probably allow to eliminate the problem. After being injected, those micro particles, nano particles and aggregates can stay around the injection site forming swellings and granulomas. But they can also be carried by the blood circulation, escaping any attempt to guess what will be their final destination. We believe that in many cases they get distributed throughout the body without causing any visible reaction, but it is also likely that, in some circumstances, they reach some organ in a fair quantity. As happens with all foreign bodies, particularly that small, they induce an inflammatory reaction that is chronic because most of those particles cannot be degraded. The protein corona effect (due to a nano bio interaction can produce organic/inorganic composite particles capable of stimulating the immune system in an undesirable way. It is impossible not to add that particles the size often observed in vaccines can enter cell nuclei and interact with the DNA.
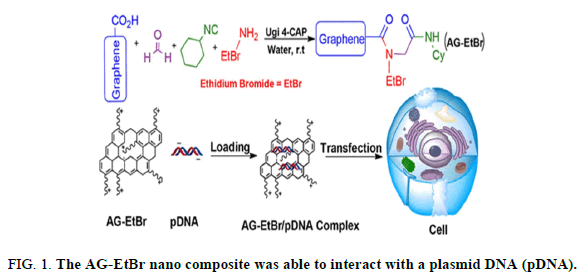
The graphene based materials with unique, versatile and tunable properties have brought new opportunities for the leading edge of advanced nano biotechnology. In this regard, the use of graphene in gene delivery applications is still at early stages. In this study, we successfully designed a new complex of carboxylated graphene (G-COOH) with Ethidium Bromide (EtBr) and used it as a nano vector for efficient gene delivery into the AGS cells. G-COOH, with carboxyl functions on its surface, in the presence of EtBr, formaldehyde and cyclo-hexylisocyanide were participated in Ugi four component reactions to fabricate a stable Amphiphilic Graphene-EtBr (AG-EtBr) composite. The coupling reaction was confirmed by further analyses with FT-IR, AFM, UV-vis, Raman, photo-luminescence, EDS and XPS. The AG-EtBr nano composite was able to interact with a plasmid DNA (pDNA) (Figure 1).
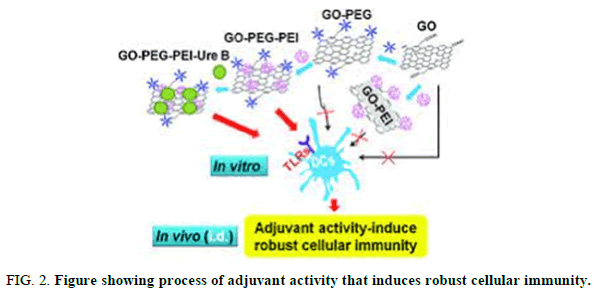
This nano composite has been applied for transfection of cultured mammalian cells successfully. The AG-EtBr composites showed a remarkable decreased cytotoxicity in compared to EtBr. The advantages of AG-EtBr in cell transfection are more dramatic (3-fold higher) than Lipofectamine 2000 as a commercial nonviral vector. To the best of our knowledge, this is the first report in which EtBr is used as an intercalating agent along with graphene to serve as a new vehicle for gene delivery application (Figure 2).
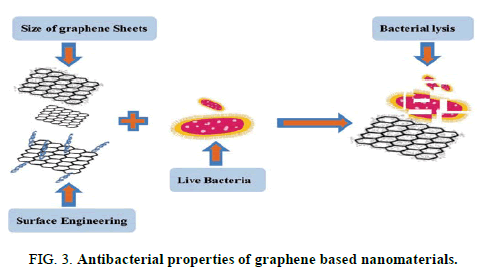
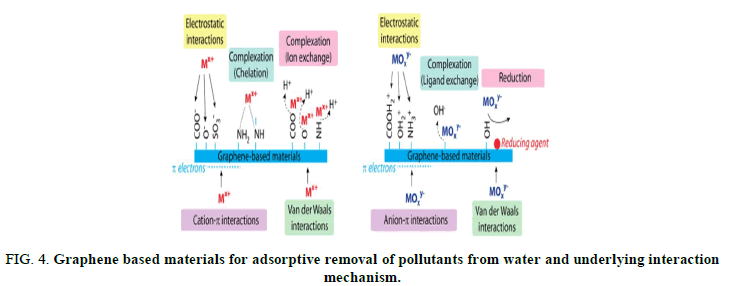
Graphene based materials have been utilized in different fields of medicine such as thermal therapy, drug delivery and cancer therapy. The prevalence of bacterial multidrug resistance MDR has attracted worldwide attention. There is a growing tendency to use nanomaterial, especially graphene family to overcome this problem. To date, no specific mechanism for antibacterial activity of graphene family has been reported. This review briefly discusses the physiochemical properties of graphene nanomaterial with a focus on the different antibacterial mechanisms, surface engineering and nano sheets size to provide a better insight for further research and development (Figures 3 and 4).
FIG 4: Graphene based materials for adsorptive removal of pollutants from water and underlying interaction mechanism.
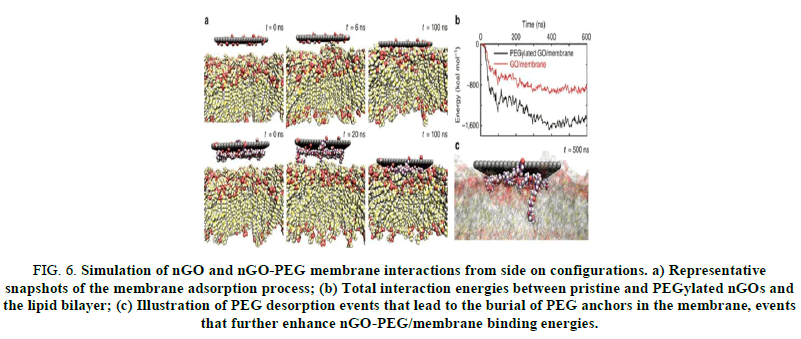
Important prerequisites for such biomedical applications involve establishing the in vivo stability and biocompatibility of nano materials in question surface passivation is widely considered to be an effective dampener of crosstalk at the bio nano interface, improving nano material stability and biocompatibility and circumventing macrophage internalization and activation. Our research works have demonstrated that PEGylation of 2D nano materials is less passivizing than previously believed. Although stable, biocompatible and non-internalized, nGO-PEG was still able to activate macrophages by triggering a potent release of cytokines. One suspect’s nGO-PEG's high available surface area for membrane interactions representative of all 2D nano materials dictates stimulatory effects on the cells in question. The simulations featured in Figure 5 support this statement: Face on nGOs and nGO-PEGs immediately attached to the membrane surface and showed no signs of desorbing (Figure 5). Though estimating quantitative binding kinetics from these simulation trajectories is not possible, total interaction energies after face on absorption favor the nGO-PEG by an approximate factor of 2. An interesting phenomenon emerges that explains this surplus nGO-PEG interaction energy: After initial face on contact, loops and termini of PEG chains desorb to form transient ‘anchors’ that bore into the lipid bilayer. One would not expect these single chain anchors to compromise membrane integrity; the additional surface area and polar moieties made available by protruding PEG molecules enhance both electrostatic and Van der Waals interactions with the membrane. Indeed, compared with pristine nGO, this augmented interaction energy is correlated with spatially tighter binding between nGO-PEG and the membrane (Figure 6) [3].
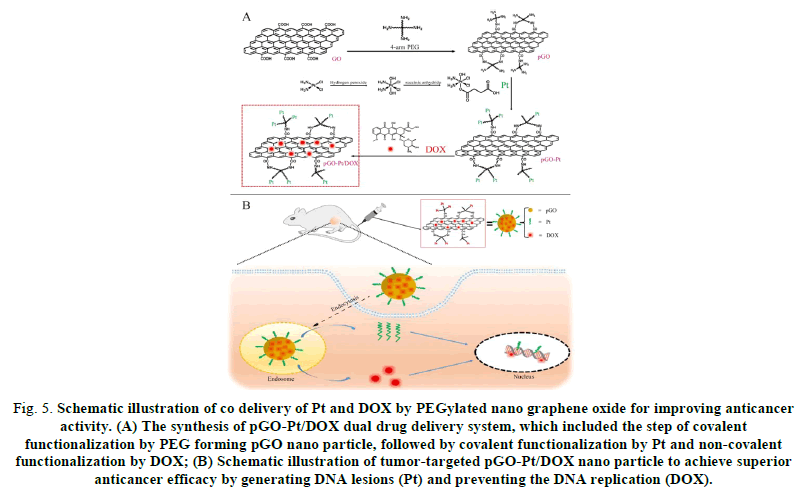
FIG 5: Schematic illustration of co delivery of Pt and DOX by PEGylated nano graphene oxide for improving anticancer activity. (A) The synthesis of pGO-Pt/DOX dual drug delivery system, which included the step of covalent functionalization by PEG forming pGO nano particle, followed by covalent functionalization by Pt and non-covalent functionalization by DOX; (B) Schematic illustration of tumor-targeted pGO-Pt/DOX nano particle to achieve superior anticancer efficacy by generating DNA lesions (Pt) and preventing the DNA replication (DOX).
FIG 6: Simulation of nGO and nGO-PEG membrane interactions from side on configurations. a) Representative snapshots of the membrane adsorption process; (b) Total interaction energies between pristine and PEGylated nGOs and the lipid bilayer; (c) Illustration of PEG desorption events that lead to the burial of PEG anchors in the membrane, events that further enhance nGO-PEG/membrane binding energies.
Graphene Oxide (GO) sheets were introduced to stabilize the melted Polyethylene Glycol (PEG) during the solid liquid phase change process, which can be used as a smart heat storage system. The structural properties and phase change behaviors of the PEG GO composites were comprehensively investigated as a function of the PEG content by means of various characterization techniques. The highest stabilized PEG content is 90 wt % in the composites, resulting in a heat storage capacity of 156.9 J g-1, 93.9% of the phase change enthalpy of pure PEG. GO has much stronger impact on lowering of the phase change temperature of PEG compared with some other porous carbon materials (activated carbon and ordered mesoporous carbon) due to the unique thin layer structure of GO. Because of the high heat storage capacity and the moderate phase change temperature, the PEG-GO composite is a promising heat energy storage candidate at mild temperature [4].
Graphene/Au composites with a high positive charge, which is advantageous for the binding and condensation of negatively charged siRNA, are synthesized via an in situ reduction method, using PEI as a reductant and protective reagent. Owing to the sufficient amounts of amino groups, PEI grafted graphene/Au composites can be further modified with methyl-PEG to acquire low cytotoxicity, novel blood compatibility and optimal dispersibility in physiological environments. The obtained PEGylated PEI-grafted Graphene/Au composites (PPGA) allow efficient loading of siRNA, forming PPGA/siRNA complexes to transport into HL-60 cells and down regulated anti-apoptosis Bcl-2 protein, indicating PPGA is a suitable platform for gene delivery. PPGA display an enhanced photo thermal response with respect to PPG under NIR laser irradiation, this suggesting that PPGA can be used as an efficient photo thermal agent.
Single stranded Ribonucleic Acid (SsRNA) acts as a probe, Antisense (AS), miRNA analog and inhibitor and is promising for gene therapy and molecular diagnosis. Free ssRNA exhibits poor cellular uptake due to its negative charges and enzyme instability, which have largely limited the practical applications of ssRNA in biomedicine. We have developed a PEGylated Reduced Graphene Oxide (PEG-RGO) nano vector for efficient delivery of ssRNA. We have demonstrated that PEG RGO exhibits superior ssRNA loading and delivery capability, compared to the widely studied PEGylated Graphene Oxide (PEG-GO). Computational simulation further suggested that PEG-RGO binds ssRNA much stronger than PEG-GO, consistent with the experimental results. These results will have implications in designing RGO-based bio compatible and efficient ssRNA delivery systems [5].
Purification of mRNA DS is vital to achieving biologically active and therapeutically administrable mRNA. The mRNA produced at lab scale can be purified using DNAses to remove DNA and subsequently isolated by lithium chloride precipitation. Multiple sophisticated DSP steps need to be employed to purify clinical grade mRNA at a large scale. Chromatography is considered a standard DSP method used by the bio pharmaceutical industry for purification processes and is widely accepted for its easy adaptability, scalability and economic feasibility. Size Exclusion Chromatography (SEC) also offers robust purification of mRNA; it is confined by its inability to separate similar sized RNA.
Similarly, ion exchange chromatography, affinity chromatography and ion pair reverse phase chromatography facilitate efficient large scale RNA purification with enhanced yield. Tangential Flow Filtration (TFF) aids in removing smaller contaminants and concentrates the DS, along with precipitation reactions, facilitating expedited purification of mRNA. The cost and efficiency of the DSP are highly dependent on the product specific purification process designed by the individual manufacturer (Figure 7).
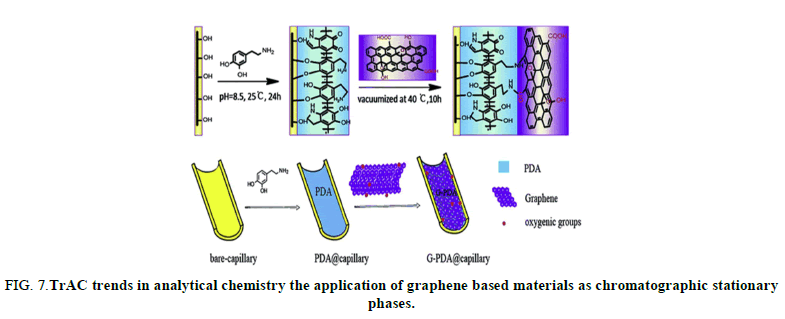
FIG 7: TrAC trends in analytical chemistry the application of graphene based materials as chromatographic stationary phases.
Graphene has fascinated the scientific researcher community since its discovery. It is a 2 dimensional sheet of sp2-hybridized carbon. It has received the attention of many researchers due to its extraordinary properties (large surface area, π-electron rich structure and good thermal and chemical stability). These remarkable properties make graphene an optimal candidate material for analytical chemistry applications. Graphene and graphene based materials has widely used in analytical chemistry, such as sample preparation, electrochemical analysis, photo chemical analysis, extraction, chemical sensing and so on. All kinds of chromatography such as Gas Chromatography (GC), Liquid Chromatography (LC) are versatile, powerful separations and analysis technique in analytical chemistry. Columns/stationary phases are considered the “heart” of the chromatograph and are responsible for the separation process (Figure 8).
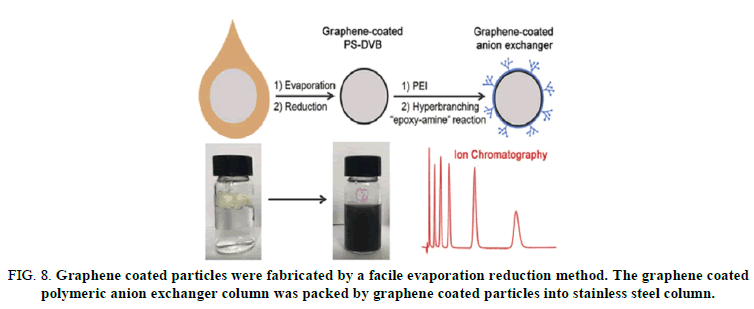
FIG 8: Graphene coated particles were fabricated by a facile evaporation reduction method. The graphene coated polymeric anion exchanger column was packed by graphene coated particles into stainless steel column.
“Carbonaceous stationary phases have gained much attention for their peculiar selectivity and robustness. Herein we report the fabrication and application of a grapheme coated polymeric stationary phase for anion exchange chromatography. The grapheme coated particles were fabricated by a facile evaporation reduction method. These hydrophilic-particles were proven appropriate substrates for grafting of Hyper Branched Condensation Polymers (HBCPs) to make pellicular anion exchangers. The new phase was characterized by zeta potentials, Fourier transform infrared spectroscopy, thermo gravimetry and scanning electron microscope. Frontal displacement chromatography showed that the capacities of the anion exchangers were tuned by both graphene amount and HBCPs layer count. The chromatographic performance of grapheme coated anion exchangers was demonstrated with separation of inorganic- anions, organic acids, carbohydrates and amino acids. Good reproducibility was obtained by consecutive injections, indicating high chemical stability of the coating”.
“With recent advances in nano technology, nano materials are being translated to applications in the fields of biosensing, medicine, and diagnostics, with unprecedented power. In this paper, we exploit the unique surface features of the bidimensional GO and its trapping ability in the presence of CA/MG ions to create a method to selectively concentrate total RNA/small RNAs in solution. The main advantage of using GOES compared to other nano particles for nucleic acid-related application is its surface to volume ratio.
One dimensional nano materials indeed possess high surface to volume ratio, which provides large active surface area for the interactions with nucleic acids, and this strongly favors the adsorption of molecules and ultimately leads to better sensitivity.
Graphene derivatives have also exceptional thermal, optical, and electronic properties that allow easy building of sensor devices. We demonstrated the feasibility of applying GO-based nanotechnologies for a highly sensitive separation of RNA-species based on size. In particular, we demonstrated that GO can enrich small RNAs” from complex solutions of RNA and how to easily detach ssNAs from GO surface”.
“The increased demand for mRNA vaccines requires a technology platform and cost effective manufacturing process with a well-defined product characterization. Large scale production of mRNA vaccines consists in a 1 or 2 step in vitro reaction followed by a purification platform with multiple steps that can include Dnase digestion, precipitation, chromatography or tangential flow filtration.
mRNA is produced in a cell free system and uses no animal derived raw materials. Cell derived impurities or adventitious contaminations are thus absent, which makes the manufacturing of these molecules safer. Although several commercial kits are available to produce mRNA for preclinical studies at laboratory scale, their costs are high. The generation of mRNA by IVT at large scale and under current good manufacturing practice (cGMP) conditions is also challenging. The specialized components of the IVT reaction must be acquired from certified suppliers that guarantee that all the material is animal component free and GMP grade. The availability of large amounts of these materials is limited and purchasing costs are high. This is true, for example, in the case of the enzymes used for translation and capping. Once the mRNA is generated by IVT, it must be isolated and purified from the reaction mixture using multiple purification steps to achieve clinical purity standards. The reaction mixture contains not only the desired product, but also a number of impurities, which includes enzymes, residual NTPs and DNA template, and aberrant mRNAs formed during the IVT. Traditional lab scale purification methods are based on DNA removal by DNAse digestion followed by Lithium Chloride (LiCl) precipitation. These methods do not allow the removal of aberrant m-RNA species such as dsRNA and truncated RNA fragments. The removal of these product related impurities is crucial for mRNA performance, as they lower translation efficiency and modifies the immune stimulatory profile. For example, a 10–1000 fold increase in protein production was observed when nucleoside modified mRNA was purified by reverse phase HPLC prior to delivery to primary DC. Chromatography is a mainstream purification process widely accepted in the pharmaceutical industry. Its high popularity is derived from several attributes such as selectively, versatility, scalability and cost-effectiveness. The first published protocol for large scale purification of synthetically produced RNA oligo-nucleotides used Size Exclusion Chromatography (SEC) in a gravity flow mode to separate molecules according to size. Further studies work applying SEC with fast performance liquid chromatography were performed. These techniques allowed preparative scale purification process, achieving high purity and high yields. SEC presents limitations, as it is not able to remove similar size impurities, such as dsDNA.
The use of Ion Pair reverse phase Chromatography (IPC) proved to be an excellent method for mRNA purification. In IPC, the negatively charged sugar phosphate backbone of the oligo nucleotides will pair with quaternary ammonium compounds QUATS present in the mobile phase to become lipophilic and then interact with the stationary phase of a reverse phase chromatography column. Elution is then performed with a gradient of an adequate solvent, (e.g., acetonitrile). Using this approach, dsRNA impurities are effectively removed while maintaining the process's high yield. IPC is challenging and costly to scale, and the use of toxic reagents such as acetonitrile, is not desirable. A new cellulose-based chromatography process for the removal of dsRNA has been described that leverages the ability of dsRNA to bind to cellulose in presence of ethanol (ETOH) . This method reported an mRNA yield of greater than 65% with a dsRNA removal of over 90%. Still, the removal of other impurities was not addressed, and thus the introduction of pre-purification steps is likely to be required.
Ion Exchange Chromatography (IEC) can also be used to purify mRNA at large scale. This technique explores the charge difference between the target mRNA species and the different impurities. For example, weak anion exchange chromatography has been successfully implemented to separate mRNA from IVT impurities. IEC presents several advantages: It is scalable and cost effective; it allows the separation of longer RNA transcripts; and it presents higher binding capacities (when compared to IPC). This chromatography must be performed under denaturing conditions. This makes the process more complex as it requires a mobile phase heater and a tight control of the temperature during chromatography.
Affinity based separation is another mRNA purification approach. A single stranded sequence of deoxythymidine (dT)-Oligo dT-is routinely used for the capture of mRNA in laboratory applications. This sequence binds to the poly-A tails present in the mRNA. Chromatographic beads with immobilized oligo dT could thus be used for the process scale purification using affinity chromatography: the poly-A tails of the single stranded mRNA produced during IVT would bind to the stationary phase while impurities are washed out. This way, IVT un-consumed reagents, the DNA template and dsRNA could be efficiently removed. While high purity products can be obtained using affinity chromatography, several drawbacks are present such as low binding capacities and a less cost-effective process.
The removal of small size impurities can also be achieved while concentrating or infiltrating solutions by Tangential Flow Filtration (TFF). Core bead chromatography can also be used for this purpose. In this case, small impurities are trapped inside the beads, and the product will be in the flow through. Both techniques rely on DNase digestion or denaturing agents to remove high size molecules such as the DNA template or the polymerase. DNA removal can also be achieved using hydroxyapatite chromatography without the use of a DNase. As a polishing step, Hydrophobic Interaction Chromatography (HIC) can be applied using connective interaction media monolith (CIM) containing OH or SO3 ligands.
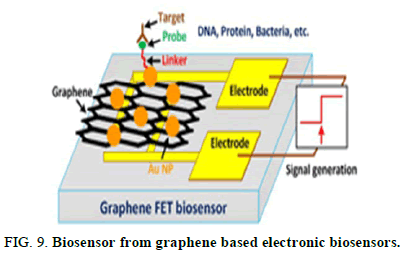
Large scale adaptations of the traditional laboratory scale mRNA purification methods are also being explored. The mRNA precipitation can be combined with TFF technique. During TFF, the membrane captures the precipitated mRNA product while other impurities are removed by diafiltration. The product is then eluted by resolubilizing the mRNA. DNA template removal can be achieved by performing the digestion with immobilized DNase. Another approach is to use tagged DNA template that can then be removed after IVT using affinity chromatography AC. Despite being scalable, these methods present a limited effectiveness since they only focus on the removal of some specific impurities and hence must be coupled with other purification steps (Figure 9).
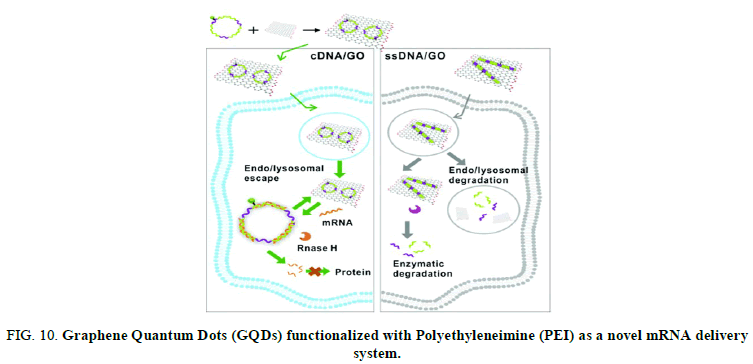
Naked, unformulated mRNA is, unable to cross the cell membrane and is susceptible to degradation. Here we use Graphene Quantum Dots (GQDs) functionalized with Polyethyleneimine (PEI) as a novel mRNA delivery system. Our results show that these modified GQDs can be used to deliver intact and functional mRNA to Huh-7 hepatocarcinoma cells at low doses and that the GQDs are not toxic, although cellular toxicity is a problem for these first generation modified particles.
A team of researchers at Fuzhou university in China have developed a graphene oxide and circularized single stranded DNA (cDNA/GO) hybrid material capable of penetrating living cells and binding mRNA (Figure 10).
FIG 10: Graphene Quantum Dots (GQDs) functionalized with Polyethyleneimine (PEI) as a novel mRNA delivery system.
Hybrid graphene oxide PEI complexes have been used for mRNA based cellular reprogramming. Graphene oxide PEI complexed with mRNA encoding the Yamanaka transcription factors facilitated the generation of iPSCs from tissue derived fibroblasts.
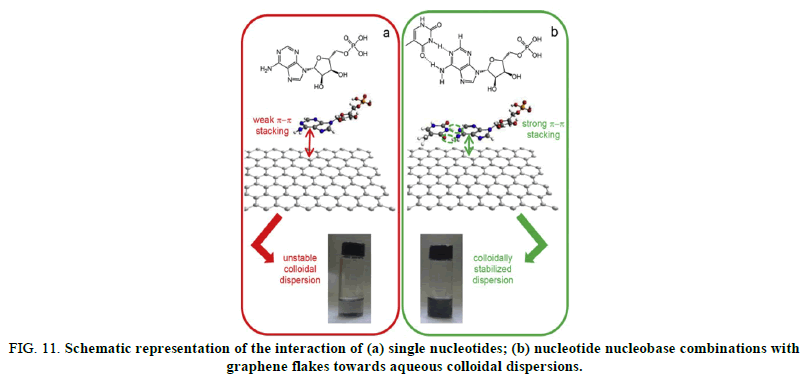
Remarkably, photo thermal effect due to the ability of graphene to absorb NIR light was suggested to enhance the transfection efficiency thanks to induced heating which locally disrupts the organization of the lipid bilayer cell membrane, hence rendering it more permeable and facilitating endosomal escape (Figure 11).
FIG 11: Schematic representation of the interaction of (a) single nucleotides; (b) nucleotide nucleobase combinations with graphene flakes towards aqueous colloidal dispersions.
For a single nucleotide, e.g. adenosine 5´-monophosphate (chemical structure given in the top part of panel a), a relatively weak interaction with the graphene surface leads to a poor colloidal stability of the flakes in water. When a nucleotide is combined with its complementary nucleobase, e.g. adenosine 5´monophosphate with thymine (shown as a hydrogen bonded cyclic dimer in the top part of panel b), the resulting supramolecular entity is able to adsorb more strongly on the graphene surface (larger area of interaction), thus enhancing the colloidal stability of the flakes in aqueous medium.
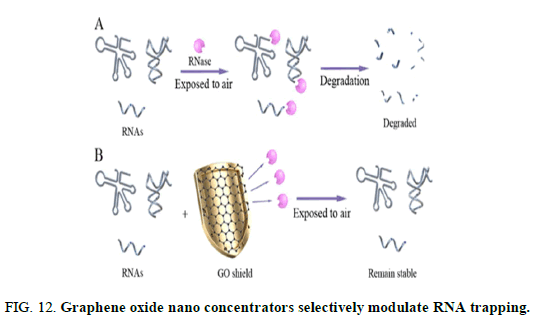
GO can be conjugated to nanomagnetic beads, defined as magnetic Graphene Oxide (GO), enabling the rapid purification and protection of the RNA from animal cells and tissues, whole blood, bacteria and plant tissue. the nano biological effects of total RNA/GO are first investigated and demonstrate that the total RNA can be harbored on the surface of GO, thus resulting in a shield effect. This shield effect allows total RNA to highly resist RNase degradation and maintain the RNA stability at room temperature up to 4 days, enabling the discovery of GO as the potential next generation RNase nanoinhibitor (Figure 12).
Materials and Methods
With an observational point of view some relevant literature and Figures 1-17 are reported to be analyzed. An experimental hypothesis project is submitted in order to provide a global conclusion related the topic of this work (Figure 13).
Results
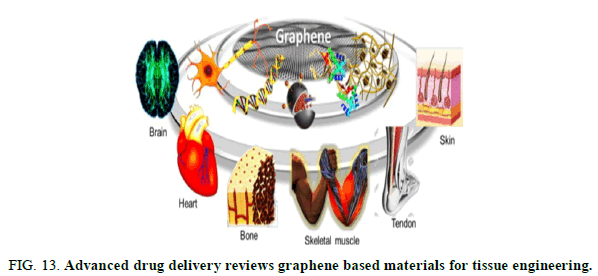
FIG 13: Advanced drug delivery reviews graphene based materials for tissue engineering.
Biopharmaceuticals main classes comprise recombinant proteins, antibodies and nucleic acid derived products, while synthetic pharmaceuticals include a wide variety of organic compounds. The purification of biopharmaceuticals, as part of downstream processing, is mainly carried out by chromatographic processes, which are responsible for the therapeutics high cost.
There is a crucial need on the development of novel and more efficient separation/purification processes or on the improvement of the current chromatographic based ones, aiming at producing pharmaceuticals of high quality and at a lower cost.
Amongst alternative techniques, it has been demonstrated that inorganic or organic nanoparticles, used in solid phase extraction approaches, may be promising for the purification of biopharmaceuticals. This chapter provides an overview on solid-liquid separation purification processes used to obtain high purity and high quality pharmaceuticals. The main purification processes are described and summarized. The areas where there has been a sustainable progress, combined with improved therapeutic characteristics are highlighted [6].
Materials based on silica nanoparticles, carbon based nanoparticles (carbon nano tubes, graphene and activated carbon) and magnetic nanoparticles are here overviewed. Based on the reported results, nanotechnology may play a key role in future pharmaceutical developments and manufacturing, where the design of suitable functionalized nanoparticles is a crucial factor to enhance the selectivity and to obtain high purification and recovery yields of bio pharmaceuticals.
Bio pharmaceuticals are a growing sector of the pharmaceutical industry. As already mentioned, biologic manufacturing or bio manufacturing is a multifaceted and complex process, comprising various steps, involving cell culture systems based on yeast or bacteria living organisms, biological complex matrices, animal and mammalian cell cultures. The main differences between synthetic drugs and bio pharmaceuticals manufacturing convey on the use of living organisms, impact of the process on the product, and complexity of the biomolecules produced. Bio pharmaceuticals are produced by recombinant DNA technology via genetically engineered living cells. For this purpose, a DNA sequence is introduced into the host cell of the living organism, which produces the target biomolecule. In the past few years, an increase in the bio pharmaceuticals manufacture from mammalian cell cultures over non mammalian systems has been observed.
The production, purification and recovery of bio pharmaceuticals require a multi step approach, in which an accurate quality control and extensive monitoring must be employed. A minor change in the process or materials, including equipment or facilities, can result in significant variations of the final product profile (molecular conformation shift) and as a consequence, more clinical tests will be required to validate the product’s safety, efficacy/purity. It is the responsibility of the manufacturer to validate, with the regulatory authorities, the impact (if any) of the changes in the manufacturing process on the bio pharmaceutical product.
The upstream process includes operations first related to cell growth in a large bioreactor system (fermenter), where the targeted bio molecule is expressed. The fermentation is developed under controlled conditions, such as pH and temperature and must be protected from the risk of contamination by other microorganisms. During the downstream processing, commonly denoted as purification process, the bio pharmaceutical is purified and isolated to obtain the final product. This is carried out through several purification steps, including filtration, chromatography and tangential flow filtration.
In the finishing operation, the final product is placed in its delivery container. The entire process is strictly regulated by FDA, EMA and/or other international regulatory agencies to guarantee the safety and efficacy of these bio based drugs. Bio pharmaceuticals are heavily regulated by authorities, which require an intensive quality control in order to comply with all regulations, as well as the efficacy and safety use guarantees. Regular quality control samples and final release specifications are conducted before approving a given batch. These specifications are controlled both by quantitative and/or qualitative approaches.
FDA and the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH) have approved the following quality elements for bio pharmaceuticals: Quality system, materials system, facilities and equipment system, production system, lab control system, packaging and labeling system.
Graphene is a carbon based material presenting a hexagonal structure composed of carbon atoms. Graphene is described as the basis of all graphitic carbon materials since it is the building block for CNTs (effectively ‘rolled up’ graphene sheets), graphite (stacked graphene sheets held together by strong van der Waals forces) and fullerene (wrapped up into graphene ball with pentagonal and hexagonal faces). Graphene presents similar and unique physical and chemical properties when compared to CNTs, such as high electrical conductivity, high surface area, adsorption capacity and thermal stability. Due to their improved compatibility and the possibility to control the surface characteristics, graphene and their derivatives are promising carbon materials for the separation, isolation and pre-concentration of drugs and biologics. Due to the large delocalized π-electron system present in the graphene framework, strong interactions to molecules containing aromatic rings occur, making them a powerful material for protein adsorption. In this way, various biomolecules have been isolated from complex matrices using graphene.
Similar work, 3D graphene/carbon nanotube aerogel was recently used for the isolation of heamoglobin. The adsorption capacity of heamoglobin was increased to 3793 mg g-1, suggesting the potential of 3D graphene for the extraction of proteins (Figure 14) [7].
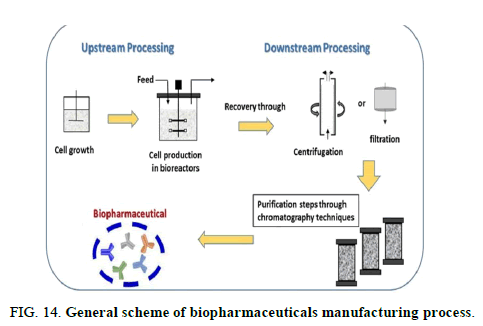
FIG 14: General scheme of biopharmaceuticals manufacturing process.
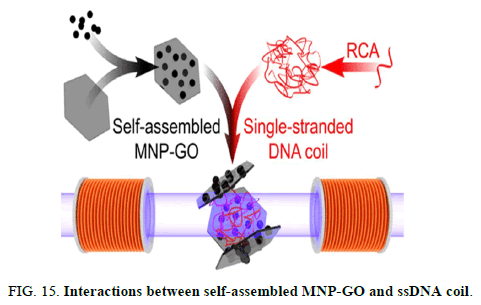
This interaction leads to a hydrodynamic size increase or aggregation of MNP-GO nano tags, which can be detected by a simple optomagnetic setup. Due to the high shape anisotropy, MNP-GO nanotags provide stronger optomagnetic signal than individual MNPs. The avoidance of DNA probes (short ssDNA sequences as the biosensing receptor) provides easier material preparation and lower measurement cost.
From real time measurements of interactions between MNP-GO and RCA products amplified from a highly conserved Escherichia coli 16S rDNA sequence, a limit of detection of 2 pM was achieved with a total assay time of 90 min. Although the nonspecific binding force between GO and ssDNA is much weaker than the specific base pairing force in a DNA duplex, the proposed method provides a detection limit similar to DNA probe based magnetic bio sensors, which can be ascribed to the abundant binding sites between GO and ssDNA. For target concentrations higher than 100 pM, the MNP-GO nano tags can be applied for a qualitative naked eye detection strategy based on nano tag ssDNA flocculation (Figure 15) [8].
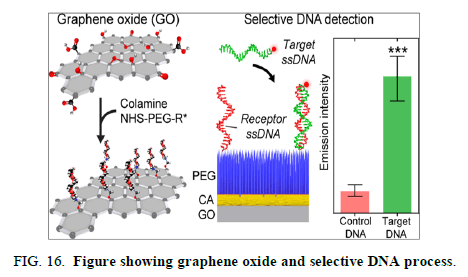
Graphene Oxide (GO) has immense great potential for widespread use in diverse in vitro and in vivo biomedical applications owing to its thermal and chemical resistance, excellent electrical properties and solubility and high surface to volume ratio. Development of GO based biological nano composites and biosensors has been hampered by its poor intrinsic bio compatibility and difficult covalent bio functionalization across its lattice. Many research studies exploit the strategy of chemically modifying GO by noncovalent and reversible attachment of biomolecules or sole covalent bio functionalization of residual moieties at the lattice edges, resulting in a low coating coverage and a largely bio incompatible composite [9].
We address these problems and present a facile yet powerful method for the covalent bio functionalization of GO using Colamine (CA) and the polyethyleneglycol cross linker that results in a vast improvement in the bio molecular coating density and heterogeneity across the entire GO lattice. We further demonstrate that our biofunctionalized GO with CA as the cross linker provides superior nonspecific biomolecule adhesion suppression with increased biomarker detection sensitivity in a DNA biosensing assay compared to the (3-aminopropyl)triethoxysilane cross linker. Our optimized biofunctionalization method will aid the development of GO based in situ applications including biosensors, tissue nano-composites, and drug carriers (Figure 16).
This capacity is mainly attributed to Carboxyl (-COOH) groups, among several oxygen functional groups, such as epoxy (C-O-C), hydroxyl (C-OH) and Carboxyl (COOH) groups. But unfortunately, the amounts of epoxide and Carbonyl (C=O) groups are known to increase with the oxidizing agent, while GO is prepared from graphite. How to increase the density of carboxyl groups at the GO surface is the major concern for the heavy metal adsorption [10]. Another advantage of GO for molecular adsorption is the high dispersibility in hydrophilic solvents and easy surface modification with other functional groups. Yang prepared GO functionalized with Lignosulfonate (LS) and Polyaniline (PANI) for the adsorption of Pb ions. The amino groups in PANI may improve the coordinate capability of sulfonic groups on LS chains and carboxyl groups on GO nano sheets for Pb ions.
Recently, the principal research trend focuses on the effects of environmental factors, such as temperature, pH and heavy metal concentration on the adsorption capacity of GO. Regretfully, studies on the structural properties of GO and competitive/selective adsorption in the presence of multiple heavy metals are insufficient. Most heavy metals HM like as Pb, Hg, Cd, Cr and As are rare metals. Although global uses of the rare metals are gradually increasing, the recycle of them has been insignificantly considered. Structural assembly of GO can facilitate the hierarchical structures with unique pore size, interlayer distance and desirable functional groups on GO. This is highly demanded to recycle those rare metals through competitive and selective adsorption/desorption of heavy metals from the distinct GO based capture system (Figure 17) [11].
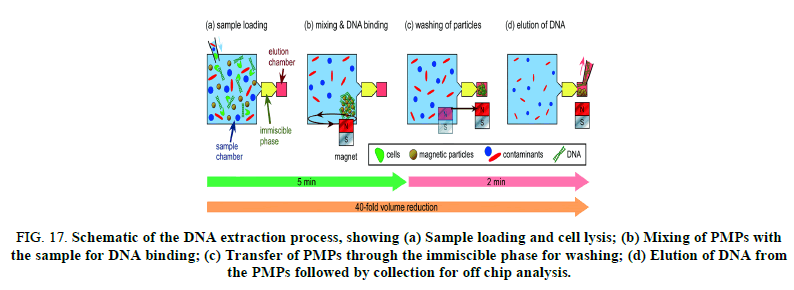
FIG 17: Schematic of the DNA extraction process, showing (a) Sample loading and cell lysis; (b) Mixing of PMPs with the sample for DNA binding; (c) Transfer of PMPs through the immiscible phase for washing; (d) Elution of DNA from the PMPs followed by collection for off chip analysis.
Among the non-viral vectors, polymer based vectors have gained great attention and are commonly used as delivery systems for mRNA based pharmaceutics. These systems are based on Nanoparticles (NPs) consisting mainly of cationic polymers that favor electrostatic interactions with the negatively charged mRNA and the cell membrane, facilitating both the encapsulation and the cellular uptake and are typically manufactured via nano precipitation or emulsion techniques. The employed cationic polymers should be bio compatible, free of toxicity as well as unwanted immunogenic effects.
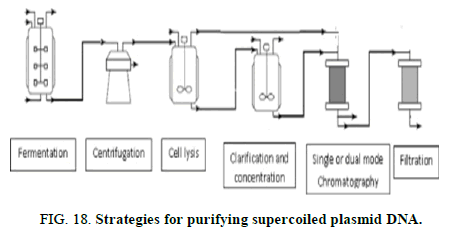
One important benefit of polymers application is their chemical flexibility and their capability to be surface modified, facilitating the targeting and controlling the release model as through pH responsive release. The typical polymers possess positively charged amino groups and can be either synthetic, such as poly-L-lysine, polyamidoamine, poly acrylic acid and poly ethyleneimine or natural, like chitosan. PEI and its derivatives are very popular cationic polymers, due to their high cationic charge density and high transfection efficiency. Polymeric materials, such as PEI have presented toxicity issues, related to high molecular weight, polydispersity and clearance or biodegradation. Many modification approaches have been tried, fabricating indicatively grafted salicylamide with PEI, Graphene Oxide (GO) PEI complexes (CHOI), micelles of conjugated PEI-2k with stearic acid, conjugated PEI with cyclodextrin, reducible polycations with histidine and polylysine residues (HIS-RPCs), poly glycoamidoamine brushes produced from poly glycoamidoamine and epoxides etc. Poly Lactic Glycolic Acid) (PLGA) nano particles have also been utilized for mRNA delivery, but with low encapsulation efficiency (Figure 18) [12].
The nucleic acid extraction method performs nucleic acid extraction by denaturing a component to be removed, such as protein, and then removing it from a solution in which a biological component is dissolved by centrifugation or the like. Therefore, since the nucleic acid to be extracted is in a state dissolved in a liquid, there is a problem that the nucleic acid is easily decomposed due to the influence of an enzyme or the like. Further, since the extraction operation repeats mixing of liquids, there is a problem that the extraction operation tends to spill.
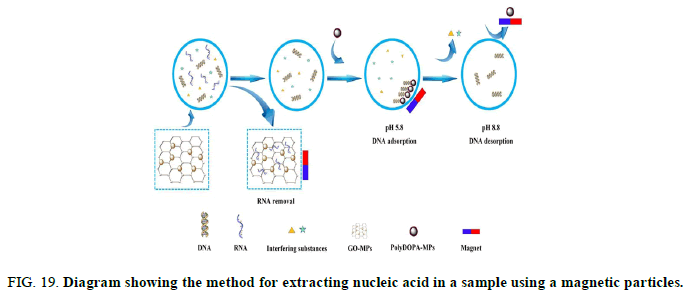
In order to solve the above problems, in 1979, a nucleic acid extraction method using solid silica particles was developed instead of the conventional nucleic acid extraction method using a liquid. In a lab, a laboratory in a university or the like sometimes recycles equipment and materials used for nucleic acid extraction for budget reasons. As a method for recycling silica particles, it is known to treat silica particles after nucleic acid extraction with HCl. As a method for extracting nucleic acid in a sample, a method using magnetic particles is also known (Figure 19) [13].
FIG 19: Diagram showing the method for extracting nucleic acid in a sample using a magnetic particles.
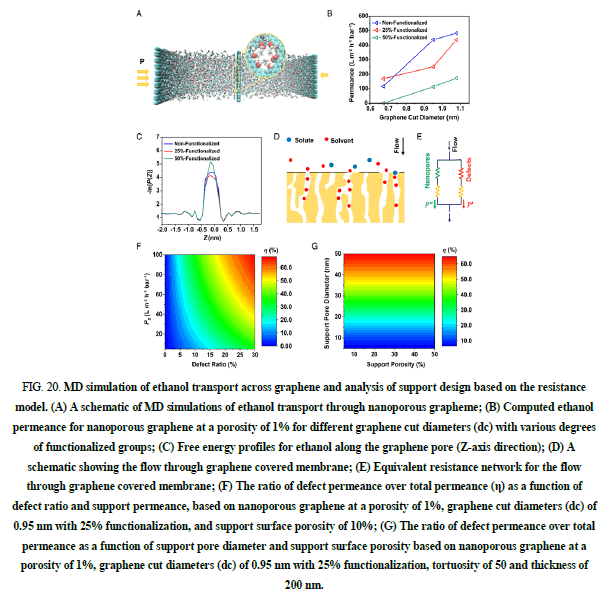
Organic Solvent Nano filtration (OSN) is an emerging technology paradigm for molecular separation in organic solvents with molecular weight cutoff ranging from 200 g mol-1 to 1,000 g mol-1. It not only has the potential to reduce up to 90% of the energy cost for solvent recovery compared with conventional evaporation or distillation processes but also, enables separation in a continuous process without biphasic or phase transition operations, thus reducing the need for large space. Due to the tremendous amounts of organic solvents involved in synthesis and cleaning, millions of tons of solvents are used in a typical Active Pharmaceutical Ingredient (API) production process (Figures 20 and 21).
FIG 20: MD simulation of ethanol transport across graphene and analysis of support design based on the resistance model. (A) A schematic of MD simulations of ethanol transport through nanoporous grapheme; (B) Computed ethanol permeance for nanoporous graphene at a porosity of 1% for different graphene cut diameters (dc) with various degrees of functionalized groups; (C) Free energy profiles for ethanol along the graphene pore (Z-axis direction); (D) A schematic showing the flow through graphene covered membrane; (E) Equivalent resistance network for the flow through graphene covered membrane; (F) The ratio of defect permeance over total permeance (η) as a function of defect ratio and support permeance, based on nanoporous graphene at a porosity of 1%, graphene cut diameters (dc) of 0.95 nm with 25% functionalization, and support surface porosity of 10%; (G) The ratio of defect permeance over total permeance as a function of support pore diameter and support surface porosity based on nanoporous graphene at a porosity of 1%, graphene cut diameters (dc) of 0.95 nm with 25% functionalization, tortuosity of 50 and thickness of 200 nm.
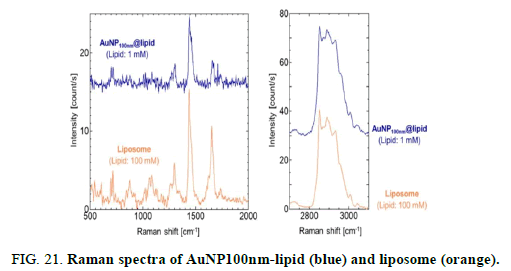
Raman spectra of AuNP100nm-lipid (blue) and liposome (orange) obtained with total lipid concentrations of 1 and 100 mM, respectively. Lipid compositions were DOPC/Chol (60/40). All samples were measured at 25°C. At least three reproducible spectra were obtained for each system. Raw spectral data are shown in the supporting information.
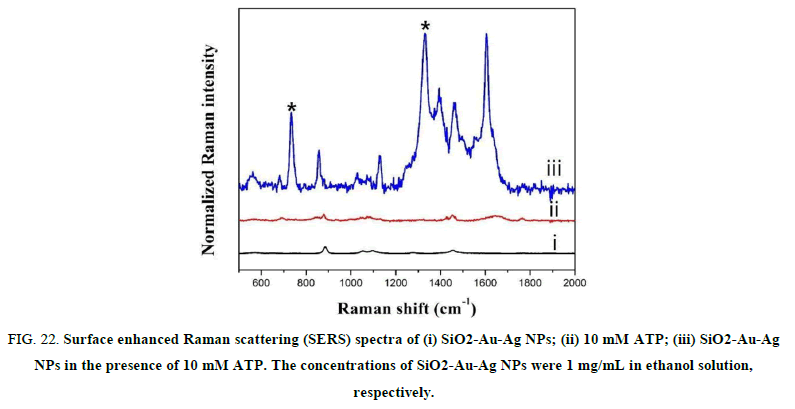
We designed and fabricated ATP encapsulated liposomes that could release ATP only when the liposome structure was ruptured for SERS based immune assays as shown in the Scheme reported. For this, ATP encapsulated liposomes and gold silver alloy (Au-Ag) assembled silica NPs (SiO2-Au-Ag) were prepared, separately. Both liposomes and SiO2-Au-Ag NPs alone were inactive for SERS measurement. When the liposome’s structure is broken and the ATP is released, a strong SERS signal could be obtained, because the released ATPs are immobilized on SiO2-Au-Ag NPs (Figure 22).
FIG 22: Surface enhanced Raman scattering (SERS) spectra of (i) SiO2-Au-Ag NPs; (ii) 10 mM ATP; (iii) SiO2-Au-Ag NPs in the presence of 10 mM ATP. The concentrations of SiO2-Au-Ag NPs were 1 mg/mL in ethanol solution, respectively.
Advances in cell engineering, process control and media composition are credited with improving the volumetric yield of cell culture bio processes, making bio pharmaceutical manufacturing more cost effective and practical. Adoption of PAT and Quality by Design (QbD) principles is an important contributor to improvements in bio process control. PAT provides real time understanding which helps to manage risk throughout a bio pharmaceutical product’s lifecycle. The PAT framework is an integrated approach using historical process knowledge, modeling and analyses [14]. Many types of physical and chemical analyses are used for bioprocessing. Traditional parameters such as pH, temperature, dissolved oxygen, feed composition and feed timing are measured in situ. Biochemical parameters such as nutrients, metabolites, amino acids, proteins, cell viability and biomass can be measured by spectroscopy, electrochemical sensors, biochemical assay or chromatography. These biochemical PATs can be used in situ, integrated with an automated sampler for at line measurements or off line. Spectroscopy PAT techniques are based on light’s interactions with materials. They provide a fast, label free, non-invasive and non-destructive chemical analysis of a material.
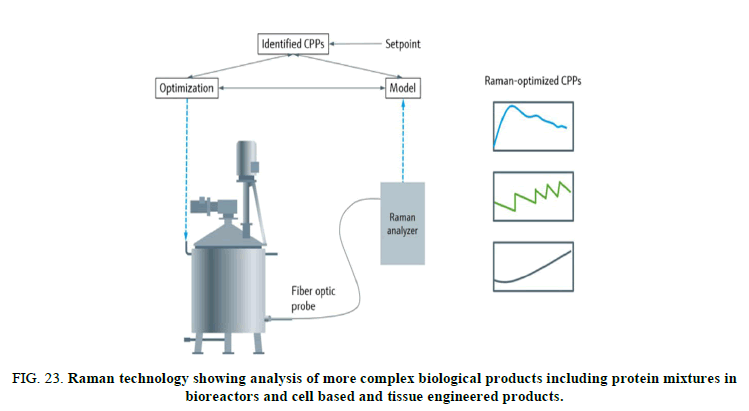
Recent technical developments in the field of Raman technology now enable manufacturers to use this technique for analysis of more complex biological products including protein mixtures in bioreactors and cell based and tissue engineered products. Raman micro spectroscopy is an inelastic light scattering based method useful for the non-destructive analysis of biochemical samples. It provides a wealth of molecular information on a specimen by the sample’s own inherent vibrational signatures. As the biochemical composition of a sample is mirrored in the Raman spectrum, mathematical methods including analytical modeling translate the physically recorded Raman data into higher level information, which can further be exploited for comparative analyses. The fingerprint like specificity of the spectral signatures can be utilized to setup a reference database of tested biological products for identification purposes (Figure 23) [15].
FIG 23: Raman technology showing analysis of more complex biological products including protein mixtures in bioreactors and cell based and tissue engineered products.
Analytical methodology: Fundamentals of the micro Raman technique due to the characteristics of the sample and to the dispersion of objects with a graphene appearance of micro metric size in a complex matrix of indeterminate composition, the direct application of spectroscopic methods does not allow characterization of the nano particles studied here without a previous microscopic localization or fractionation from the original sample [16]. Therefore, microscopy coupled to Raman spectroscopy (micro-Raman) was selected as an effective technique for an exhaustive screening of micrometric objects visible under the optical microscope.
Discussion
Analytical methods have been adequately described. In the description of the HPLC purity method information on the reporting threshold is missing and should be provided post approval. Validation data for the analytical methods to control DMG-PEG2000 are missing and should be provided post approval (REC). Batch analysis data for five batches have been provided. All results comply with the specifications. More detailed information on these batches will be provided post approval (REC). Some clarifications and amendments are needed for the manufacturing process description (REC). Some clarifications and amendments were needed for the manufacturing process description [17]. Respective information and/or commitments have been provided.
The proposed CQAs of the LNP are appearance, mRNA identity, total RNA content, purity and product related impurities, % RNA, particle size, lipids identification, lipids content, lipids purity, pH, osmolality, bacterial endotoxins and bio burden. The CPPs for the mRNA-1273 LNP manufacturing process have been described. No CIPCs were identified for the mRNA-1273 LNP manufacturing process. A risk assessment concerning potential extractable and leachable from manufacturing components and container closure systems has been performed according to the applicant. No details are available on possible extractable. The applicant will provide respective data (REC).
According the European pharmacopeia, among the methods established for quality control of classical medicines the so called “non-invasive”, e.g. non-destructive, techniques, such as near infrared and Raman spectroscopy have been applied for molecular imaging and analytics in Process Analytical Technology (PAT) and are implemented in Quality by Design (QbD) concepts.
Conclusion
Many process and biotechnology use graphene derivates product in example pharmaceuticals purifications, in purifying extracting RNA or related the property of absorption, carrier, adjuvant to increase RNA stability, magnetic micro bead for improve extractions procedures. Observing all this reported in references it is needed that regulatory agency as mandatory order require specific written report related certification about graphene derivates absence in the final biopharmaceutical products (every batch/lot) and this also for the raw material: it must to be certified and reported in the technical and security sheet and this even if are present patent and other rights for the industrial pharmaceuticals producers. The fact that a new technology provide great advantages in efficiency of production not mean that the safety and security criteria it not must be followed. To make of full knowledge by the public opinion of this fact make possible to share to all a wider safety perception useful in the vaccination strategy.
References
- Pham XH, Baek A, Kim TH, et al. Graphene oxide conjugated magnetic beads for RNA extraction. Chem Asian J. 2017;12(15):1883-1888.
[Crossref] [Google Scholar] [PubMed]
- Kainz QM, Reiser O. Polymer and dendrimer coated magnetic nanoparticles as versatile supports for catalysts, scavengers and reagents. Acc Chem Res. 2014;47(2):667-677.
[Crossref] [Google Scholar] [PubMed]
- Tian B, Han Y, Fock J, et al. Self-assembled magnetic nanoparticle graphene oxide nano tag for optomagnetic detection of DNA. ACS Appl Nano Mater. 2019;2(3):1683-1690.
- Yun T, Jeong GH, Padmajan Sasikala S, et al. 2D graphene oxide liquid crystal for real world applications: Energy, environment and antimicrobial. APL Mater. 2020;8(7):070903.
- Palmieri V, Di Pietro L, Perini G, et al. Graphene oxide nano concentrators selectively modulate RNA trapping according to metal cations in solution. Front Bioeng Biotechnol. 2020;8:421.
[Crossref] [Google Scholar] [PubMed]
- Hashemi E, Akhavan O, Shamsara M, et al. DNA and RNA extractions from eukaryotic and prokaryotic cells by graphene nanoplatelets. RSC Adv. 2014;4(105):60720-60728.
- Ouranidis A, Vavilis T, Mandala E, et al. mRNA therapeutic modalities design, formulation and manufacturing under pharma 4.0 principles. Biomedicines. 2021;10(1):50.
[Crossref] [Google Scholar] [PubMed]
- Young RO. Scanning and transmission electron microscopy reveals graphene oxide in COVID-19 vaccines. Acta Sci Med Sci. 2022;6(8).
[Crossref]
- Luisetto M, Almukthar N, Tarro G, et al. Graphene and derivates: Physicochemical and toxicology properties in the mRNA vaccine manufacturing strategy. Sci World J Pharm Sci. 2022;1(2):1-23.
- Luisetto M, Edbey K, Tarro G, et al. Relevant article, white papers and other documents concerning the m RNA vaccine: An interesting collection useful to better understand some phenomena and to generate hypothesis. Int J Forensic Res. 2022;3(2):129-145.
- Jeon KY. Moving and living microorganisms in the COVID-19 vaccines prevention, early treatment cocktails for COVID-19 and detoxification methods to reduce sequels of COVID-19 vaccines. Am J Epidemiol Public Health. 2022;6(1):1-6.
- Vanden-Hehir S, Tipping WJ, Lee M, et al. Raman imaging of nanocarriers for drug delivery. Nanomaterials. 2019;9(3):341.
[Crossref] [Google Scholar] [PubMed]
- Lee YM, Park S, Jeon KY. Foreign materials in blood samples of recipients of COVID-19 vaccines. Int J Vaccine Theory Pract Res. 2021;2(1):249-265.
- Xu H, Qi Z, Jin H, et al. Identification of graphene oxide and its structural features in solvents by optical microscopy. RSC Adv. 2019;9(32):18559-18564.
[Crossref] [Google Scholar] [PubMed]
- Agieienko V, Neklyudov V, Dimiev A. Solvent induced changes in the graphene oxide absorption spectrum. The case of dimethylsulfoxide/water mixtures. J Mol Liq. 2019;287:110942.
- Yang K, Chen B, Zhu X, et al. Aggregation, adsorption and morphological transformation of graphene oxide in aqueous solutions containing different metal cations. Environ Sci Technol. 2016;50(20):11066-11075.
[Crossref] [Google Scholar] [PubMed]
- Tarro G. Environment and virus interactions: Towards a systematic therapy of SARS-CoV-2. Br J Healthcare Med Res. 2022;9(4).