Research
, Volume: 18( 1)Batch Electrochemical Coagulation (BECC) of Domestic Wastewater and Grey water for the Removal of COD and TDS
- *Correspondence:
- Megha Subramanya Department of Civil Engineering, Maharaja Institute of Technology, Mysore, India. Tel: +918884711426; E-mail: megha.subramanya7@gmail.com.
Received: December 27, 2021, Manuscript No. TSES-21-39107; Editor assigned: January 03, 2022, Pre QC No. TSES-21-39107(PQ); Reviewed: January 13, 2022, QC No. TSES-21-39107; Revised: January 24, 2022, Manuscript No: TSES-21-39107(R); Published: February 01, 2022, DOI: 10.37532/environmental-science.2022.18.206
Citation:Megha Subramanya. Batch Electrochemical Coagulation (BECC) of Domestic Wastewater and Grey water for the Removal of COD and TDS. Environ Sci Ind J. 17(12):206
Abstract
Rapid urbanization has increased the problem of the discharge of untreated wastewater into the environment and is a major concern worldwide. Since wastewaters contain toxic pollutants and contaminants which pollute the receiving water bodies, the removal of such pollutants and contaminants from wastewaters has recently become the subject of considerable interest to control the deviating effect of water pollution. Depending on the origin and composition, domestic wastewater can be subjectively segregated into grey water and black water. Grey water generally includes sources from bathtubs, showers, hand washing basins, washing machines, dishwashers and kitchen sinks which is considered as a major part of the overall household wastewater with the share of 50%-80%. Wastewater originating from the toilets (water, urine and faeces) is known as black water, containing high amount of pathogens and organic load that poses the highest risk of biggest contamination. Grey water treatment is much easier than black water because black water is severely contaminated. The composition of the grey water mainly depends on family size, lifestyle of the people, washing habits and type of the detergents used for dishwashing and washing machines. In this research work, the objective was to treat domestic wastewater and grey water for the removal of COD and TDS using batch electrochemical coagulation, using stainless steel electrode. The specific objectives focused on characterization of domestic wastewater and grey water for various physico-chemical parameters and BECC was set to arrive at the conditions for the removal of COD and TDS using stainless steel, electrodes and operating parameters such as cell voltage, current density, electrode spacing and electrolysis time; and to observe the influence of water quality parameters on electrochemical coagulation process with fish survival studies at different dilutions.
Introduction
Due to rapid urbanization the problem of the discharge of un-treated wastewater into the environment has been of major concern
World-wide. Since wastewaters contain toxic pollutants which pollute the receiving water bodies, the removal of those pollutants from wastewaters has recently become the subject of considerable interest to control water pollution [1].
Domestic wastewater, by its origin and composition can be segregated into grey water and black water. Grey water is the urban wastewater, generally includes sources from bathtubs, showers, wash basins, washing machines and kitchen sinks which is considered as a major part of the overall household wastewater with the share of 50%-80%. Wastewater originating from the toilets (water, urine and faeces) is known as black water, which contain high amount of pathogens and organic load that poses biggest contamination risk. In India there is no separation of greywater and blackwater. Henceforth, both greywater and blackwater is collected and treated together. Greywater treatment is much easier than blackwater because blackwater is more contaminated. Greywater composition mainly depends on family size, lifestyle of the people, washing habits and type and quantity of detergents used for dishwashing and washing machines [2].
Discharge of untreated domestic wastewater lead to pollution of groundwater sources by nutrients and micro-pollutants, production of offensive odor eutrophication of surface water bodies, pollution of groundwater and causes soil salinity [3].
Total Suspended Solids (TSS) in wastewater contributes to the sedimentation and reduction of the hydraulic capacity of drainage channels and surface water reservoirs, and causes clogging of the filter media systems. Domestic wastewater contains oil and grease in which reduces the soil’s ability to transmit water and efficiency of treatment by interfering with the physical, chemical and biological processes. Excess nutrients may leach through the soil to enter waterways and aquifers resulting in algal blooms or came other water quality issues. Some detergents and optical brighteners are slow to biodegrade which may persist in the environment for long periods of time. Domestic wastewater contains waterborne viruses, bacteria, parasitic, protozoa etc. which cause diseases. Soaps add pollutants to water during washing such as sulphates, chlorides which forms scum in hard waters. The cleaning agents contain sodium salts which, if present in excessive amounts damage soil structure create an alkaline condition and also increase the SAR. To protect public and environmental health improved domestic wastewater collection and proper treatment is necessary.
Methodology
The composition and characteristics of the domestic wastewater vary hourly, diurnally and seasonally and with age. Recalcitrant and non-refractory compounds present in the wastewater vary from source to source, lifestyles, customs, and use of chemicals in households. Domestic wastewater was collected from the sewage treatment plant, Kesare, Mysore [4].
The greywater samples used in this research is the Kitchen Wastewater (KWW) [5]. KWW is the wastewater generated after washing utensils, dishes, vegetables washing etc. KWW were collected in polypropylene containers as and when required for analysis and BECC. The duration of the sample storage before starting experiments varied from one day to four weeks. KWW used in lab scale experiments were collected from Hotel Mylari, SJCE Campus, Mysuru. The composition of KWW depend on factors viz., number of consumers, type of food served, quantity and quality of water used, peak hours and use of cleaning/sanitizing agents for hygiene. Raw domestic wastewater and kitchen wastewater so collected were characterized for parameters such as pH, Chemical Oxygen Demand (COD), Total Dissolved Solids (TDS), conductivity, nitrates, phosphates, total alkalinity, total hardness etc.
Determined according to the standard methods for examination of water and wastewater (APHA 2005). PH and conductivity was measured using digital pH meter and conductivity meter respectively. Nitrates, phosphates and sulphates were determined using Spectrophotometer. TDS was determined by gravimetric method. Chlorides were determined by Argent metric-Titrimetric method. Total alkalinity and total hardness were determined by titrimetric method. COD was titrimetrically determined by using Closed Reflux method.
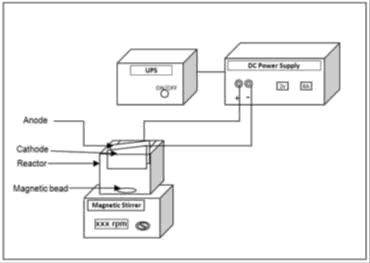
BOD5 was measured by Azide modification method. BECC experiments were carried out in a cubical shaped acrylic glass reactor with 1L capacity with arrangements to hold 1mm thick, 100 mm x 50 mm size plate as electrodes made of stainless steel, copper and iron. DC power supply was used as a power source for experiments to maintain the required current and voltage across the electrodes. An Uninterruptible Power Supply (UPS) was used to maintain a continuous supply of electric power to the BECC system in the event of power failure. Magnetic stirrer with magnetic bead was used to maintain homogeneity of the bulk solution [6].
Experimental Approach
A lab scale Electrochemical Reactor (ECR) of 1 L capacity was fabricated and used for the experiments. The electrodes stainless steel, copper and iron were washed to remove the impurities present on the electrode surface. Then the initial weight of each of the electrodes was determined. The electrodes were placed in the reactor vertically with 1cm inter-electrode spacing. With the magnetic bead placed at the bottom the reactor was filled with 1 L of wastewater. The electrodes were connected to the DC power supply with positive terminal connected to anode and negative terminal to cathode. The reactor was kept under completely mixed condition by means of magnetic stirrer with the stirring speed of suitable rpm. Most experiments were carried out by varying the cell voltage, current and also changes in SA/V. The samples were collected from the reactor at regular time intervals, filtered and analysed for COD and ph. All the BECC experiments were conducted at room temperature [7]. After noting the performance of the electrodes used and observing the results obtained, it was seen that the maximum removal of COD and TDS was achieved while using stainless steel electrodes for different operating conditions, cycle studies were carried out. To study the influence of water quality parameters on electrochemical coagulation process, fish survival tests were conducted with different dilutions.
Experimental setup and procedure
The schematic experimental setup consisting of a single cell batch reactor, electrodes, magnetic stirrer, magnetic bead, power supply
Results and Discussion
DWW used for Batch Electrochemical Coagulation (BECC) was collected from the Sewage Treatment Plant (STP) located in Kesare, Mysuru. Kesare STP is one of the three STPs functioning in Mysore and has been selected due to its proximity to the industrial estate. The Kesare STP is spatially spread over around 8.1 Ha. The Kesare STP is designed for an ultimate treatment capacity of 30 MLD. At present, the STP is getting utilized to treat only around 20 MLD of sewage. The STP is operated using Facultative Aerated lagoons process of treatment. KWW used for batch ECC was collected from Hotel Mylari, SJCE Campus, and Mysuru. The primary contribution to the KWW is from wash sinks from kitchens. Water used for cleaning vegetables, washing dishes and cooking utensils, returns as wastewater. The wide variety of food served vary from time to time for a particular restaurant and are expected to be different from one restaurant to other, the wastewater composition also varies. Wastewaters generated from restaurant not only differ by volume but also change in characteristics [8].
Characteristics of domestic wastewater and kitchen wastewater
The DWW comprises of both greywater and black water. TABLE 1 shows the physico-chemical characteristics of both DWW and KWW.KWW samples were collected as and when required for the BECC experiments and were analysed for pH, conductivity, TS, TDS, chlorides, total alkalinity, total hardness, COD, nitrates, phosphates and sulphates etc. in accordance with the Standard methods (APHA 2005). Wastewater characteristics depend on the load, cooking ingredients and use of different cleaning agents and left over wastes as well. The cleaning agents used were a mixture of soap oil (1 L), caustic soda (sodium hydroxide, NaoH) (50 g), washing soda (sodium carbonate, Na2CO3) (50 g) and urea (CH4N2O) (100 g).
Table 1. Characteristics of domestic waste water and kitchen waste water.
| Parameter | Unit | DWW | KWW |
|---|---|---|---|
| Flow | m3d-1 | 20000 | 1000-1200 |
| Cleaning agents | - | - | Soap oil+ NaOH+Na2CO3+CH4N2O |
| Color | - | Greyish green | Yellowish orange |
| PH | - | 6.4-8.0 | 6.48-8.5 |
| Chloride | mgL-1 | 90-190 | 170-230 |
| Total solids | mgL-1 | 1550-1880 | 1200-3600 |
| conductivity | µScm-1 | 1200-1600 | 1400-1800 |
| COD | mgL-1 | 700-1800 | 800-2080 |
| Total dissolved solids | mgL-1 | 880-1200 | 900-2080 |
| Total alkalinity as caco3 | mgL-1 | 250-300 | 330-700 |
| Total hardness as caco3 | mgL-1 | 340-450 | 350-470 |
| Nitrate | mgL-1 | 15-30 | 10-45 |
| Phosphate | mgL-1 | 20-45 | 5-42 |
| Sulfate | mgL-1 | 10-40 | 15-75 |
Table 1 shows relatively larger COD values, nitrate, phosphates and sulphates present in domestic wastewater because of nutrients, dissolved salts, cleansing solvents, detergents, human faeces and urine. Conductivity value is rather higher because of salts of Ca, Mg, K, and Na present. The composition and characteristics of the domestic wastewater vary hourly, diurnally and seasonally and with age.
BECC of Domestic Waste Water (DWW)
DWW was collected from the equalization tank of a STP located in Kesare, Mysuru. It was treated by BECC using different electrode materials in a 1 L ECR for cell voltages of 5, 7 and 10 V with the corresponding current density of 0.05, 0.08, 0.1 a respectively.
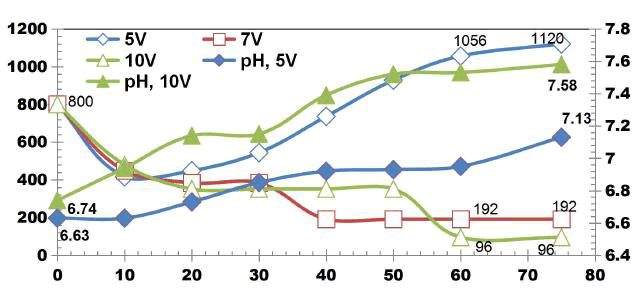
BECC Stainless steel electrodes: FIG. 2 shows COD degradation values for DWW for cell voltages of 5, 7, 10 V using four SS electrodes with the corresponding current density of 0.05, 0.08, 0.1 A. The initial COD value of DWW was 800 mgL-1. Irrespective of applied voltages, unusual fluctuations in COD values was observed during treatment due to the recalcitrance nature and fluctuations in the current across the electrodes. COD removal efficiencies of 88% and 76% were observed at the 75 min ET for cell voltages of 10 V and 7 V respectively. The results show that the maximum removal of COD from 800 KmgL-1 to 96 mgL-1. Also the electro-dissolution of coagulants increased as the current density increases.
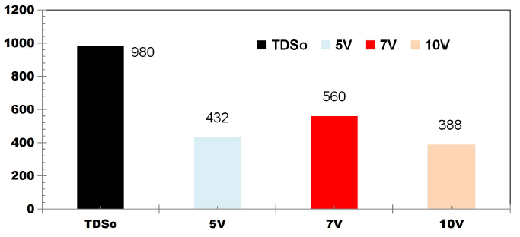
FIG. 3 shows change in TDS values for DWW for cell voltages of 5, 7 and 10V using four SS electrodes with the corresponding applied current of 0.05, 0.08, 0.1 A. The initial TDS value of DWW was 980mgL-1. The TDS removal of 55.91%, 42.85% and 60.41% were observed for applied cell voltages of 5, 7 and 10 V respectively while using four SS electrodes.
BECC Stainless steel electrodes for changing SA/V: After noting the performance of SS electrodes in sub-section 4.3.1, it was felt sufficient to keep HRT at 60 min for further experiments that followed. A series of BECC experiments were carried out by increasing the electrode pairs by 2, 4, 6 and 8 respectively for a fixed applied potential of 10V for the corresponding current of 0.2 A. The experiments were carried out under the operating conditions of ECR: 1 L; ET: 75 min; electrode spacing: 1 cm; agitation speed: 350 rpm. It was observed that on every addition of electrode pair in each experiment for a fixed cell volume of 1 L, a drop in current was observed across the electrodes during treatment.
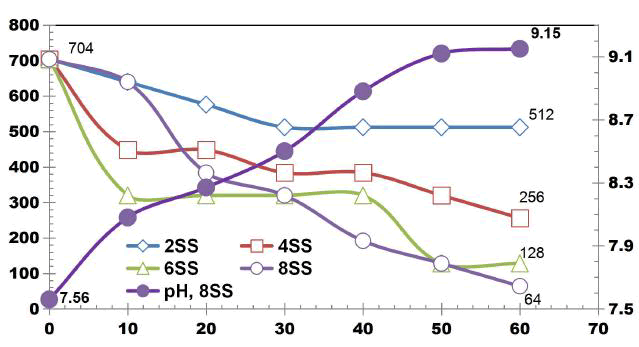
FIG. 4 illustrates the COD removal for cell voltage of 10 V by using SS electrodes for change in SA/V. COD removal efficiency of 90.91% was achieved using 8 SS electrodes, similarly 27.27%, 63.63% and 81.81% COD removal was observed at 75 min ET using 2SS, 4SS and 8SS electrodes respectively [9].
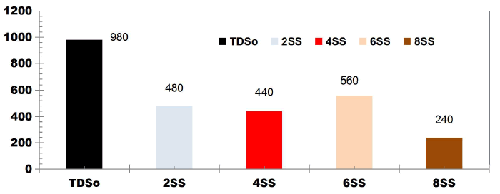
FIG. 5 describes the TDS removal as a function of ET for DWW using SS electrodes for change in SA/V at cell voltage of 10 V. The TDS removal efficiency at 75 min ET was 51.02%, 55.10%, 42.85% and 75.51% using 2SS, 4SS, 6SS and 8SS electrodes respectively.
FIG. 5. TDS removal as a function of ET for DWW using SS electrodes for change in SA/V at cell voltage of 10 V.
BECC of Kitchen Waste Water (KWW)
The KWW was subjected to BECC using SS electrodes and the results are presented in the following sub-section.
BECC Stainless steel electrodes: The BECC experiments were carried out in an ECR of 1L volume. The operating conditions were 4 E: SS; electrode spacing of 1cm; agitation speed of 350rpm; cell voltages: 8, 12 and 16 V; ET: 75 min; CODo: 1120 mgL-1; TDSo: 2080 mgL1; pHo: 6.12.
FIG. 6 illustrates the COD degradation as a function of ET at different voltages using four SS electrodes for applied voltages of 8V, 12 V and 16 V. The initial COD value of raw wastewater was 1120 mgL-1.
COD gradually decreases as electrolysis proceeds with COD removal efficiencies of 71. 42% at the end of 75 min ET at applied voltage of 8 V and 12 V. At 16 V, 85.71% of COD removal was observed.
FIG. 6. COD degradation as a function of ET at different voltages using four SS electrodes for applied voltages of 8 V, 12 V and 16 V.
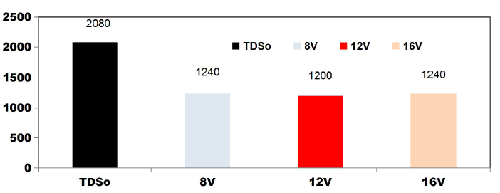
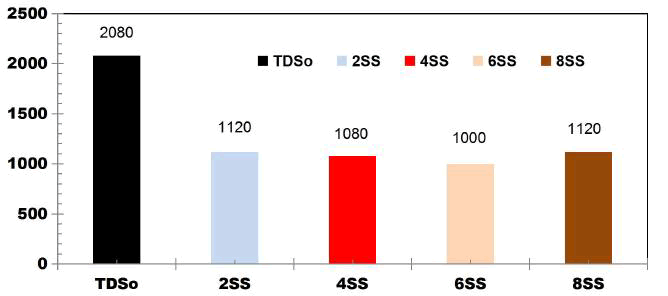
FIG. 7 shows the reduction in TDS value after 75 min for different applied cell voltages of 8, 12 and 16 V while using four SS electrodes. With the initial TDS value of 2080 mgL-1, for 8 V the TDS removal efficiency is 40.38%, like wise for 12V and 16V, TDS removal efficiency was 42.3% and 40.38% respectively.
FIG. 7. Reduction in TDS value after 75min for different applied cell voltages of 8, 12 and 16V while using four SS electrodes.
BECC SS electrodes for changing SA/V: After observing the performance of SS electrodes, it was felt sufficient to keep HRT at 75 min for further experiments that followed.
A series of BECC experiments were carried out by increasing the electrode pairs by 2, 4, 6 and 8 respectively for a fixed applied potential of 16 V with the corresponding current densities of 1.3 A, 0.4 A, 0.3 A & 0.2 A. The experiments were carried out under the operating conditions of ECR: 1 L; ET: 75 min; electrode spacing: 1 cm; agitation speed: 350 rpm.
FIG. 8 describes the COD reduction as a function of ET for cell voltages of 16 V by varying number of electrodes.
COD removal efficiency is higher in lesser number of electrodes. As the number of electrodes increases current gets distributed. Using 2SS COD removal efficiency of 87.5% was observed. Similarly 50% COD removal was achieved using 4SS and 6SS electrodes. For 8SS, 75% COD reduction was observed. When the number of electrodes increases the efficiency of COD removal decreases slightly because the rate of electron transfer becomes slower [10].
FIG. 9. TDS reduction after 75 min ET for applied cell voltage of 16 V using 2SS, 4SS, 6SS and 8SS electrodes.
FIG. 9 shows the TDS reduction after 75 min ET for applied cell voltage of 16 V using 2SS, 4SS, 6SS and 8SS electrodes. Using 2SS and 8SS electrodes 46.15% TDS removal efficiency was observed. 48.07% TDS removal efficiency was achieved using 4SS electrodes at the end of 75 min ET. While using 6SS, TDS removal efficiency of 51.92% was achieved at 16 V after ET of 75 min.
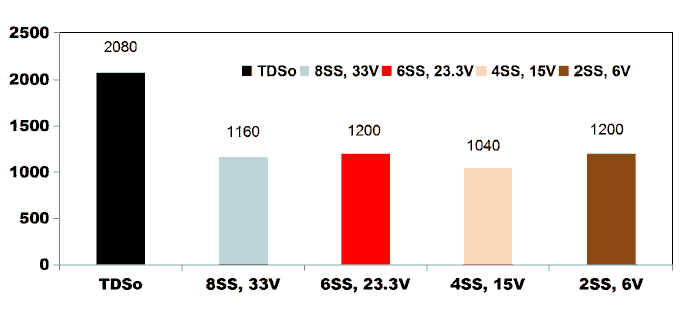
FIG. 10 shows the COD degradation curve as a function of electrolysis time for kitchen wastewater for applied current of 0.4 A by varying number of electrodes. For an applied current of 0.4 A with the corresponding cell voltage of 33 V and 15 V, using 8SS and
4SS electrodes, the COD removal efficiency of 71.28% was observed. 85.71% of COD removal was achieved using 2SS and 6SS electrodes for the cell voltage of 23.3 V and 6 V [11].
FIG. 10. COD degradation curve as a function of electrolysis time for kitchen wastewater for applied current of 0.4 A by varying number of electrodes.
FIG. 11. Reduction in TDS value after ET of 75 min by varying number of electrodes for an applied current of 0.4 A.
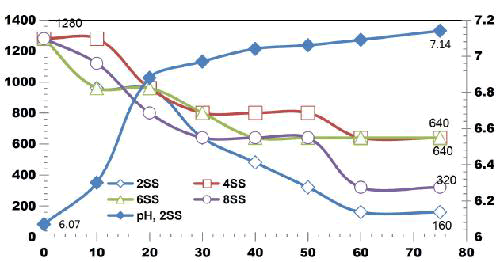
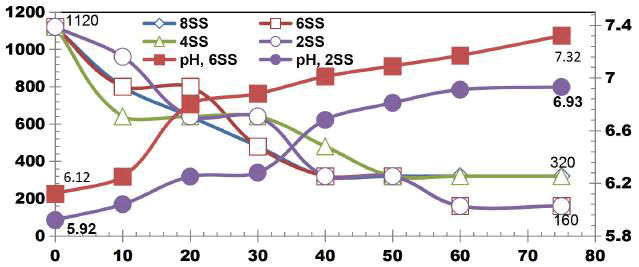
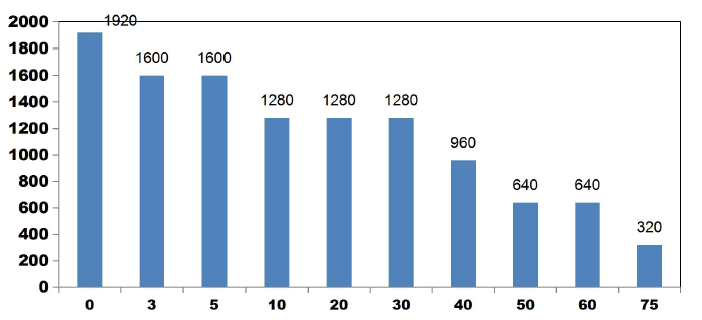
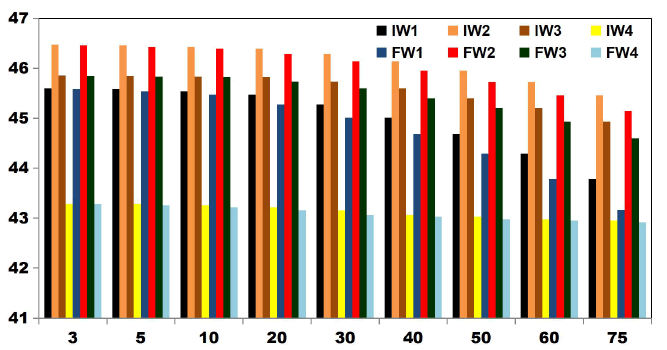
FIG. 11 shows the reduction in TDS value after ET of 75 min by varying number of electrodes for an applied current of 0.4 A. 42.3% of TDS reduction was observed when using 2SS and 6SS electrodes. While using 8SS and 4SS electrodes, 44.23% and 50% of TDS removal efficiency was achieved [12]. BECC (Using stainless steel electrodes by varying electrolysis time (ET)): BECC experiments were carried out for discrete ETs for KWW using SS electrodes. Fig. 4.19 shows the COD removal for KWW for a fixed applied cell voltage of 16 V and corresponding current of 0.5 A for discrete ETs of 3, 5, 10, 20, 30, 40, 50, 60 and 75 min.
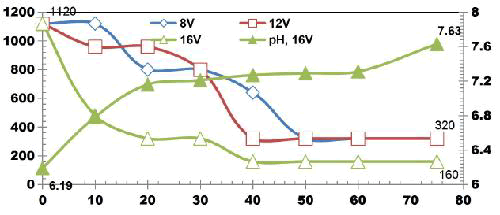
FIG. 12 also explains how COD values reduce with discrete in ETs. The initial COD value of raw KWW was 1920mgL-1. COD gradually decreases as electrolysis proceeds with COD removal efficiencies of 16.66% for 3 and 5min. 33.33% of COD removal was observed at 10, 20 and 30 min. 66.66% COD removal efficiency was achieved at 50 min and 60 min. 50% and 83.33% COD reduction was observed at 40 min and 75 min respectively.
COD removal was seen to follow a step function showing intermediate long stability time and short maturity time in the electrochemical reactor as also observed by Alex and Paul, 2015, India.
FIG. 13. TDS reduction as a function of ET for KWW for applied cell voltage of 16 V for the corresponding current of 0.5 A.
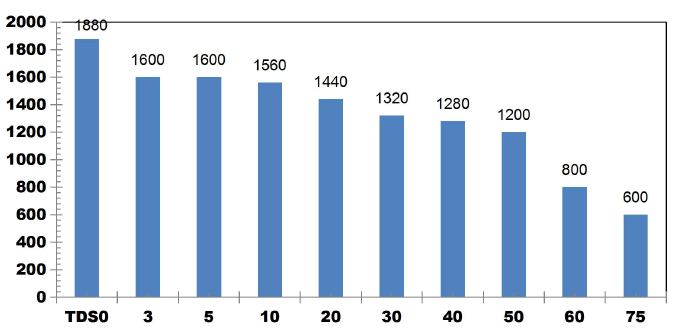
FIG. 13 shows the TDS reduction as a function of ET for KWW for applied cell voltage of 16 V for the corresponding current of 0.5 A for the electrolysis time of 3, 5, 10, 20, 30, 40, 50, 60 and 75 min. The removal efficiency of TDS for 16 V, 0.5 A at 3 and 5 min is 14.89% and at 10 min 17.02% TDS removal was achieved. 23.40%, 29.78%, 31.91%, 36.17%, 57.44% and 68.08% of TDS removals were obtained for ETs of 20, 30, 40, 50, 60 and 75 min respectively.
TDS was seen to be in control after 60 min ET showing the stability of the process [13].
FIG. 14 illustrates the electrode dissolution using 30 m2m-3 SS electrodes for KWW. As seen the graph, the electrode dissolution increases as the electrolysis time increases. Electrode dissolution depends on the position when arranged. Electrode loss is due to the generation of metal ions within the bulk solution during ECC and these metal ions are later precipitated [14-15].
Fish Survival Test
BECC for KWW was conducted using SS electrodes with a fixed applied cell voltage of 16 V for 75 min ET. Post ECC supernatant was filtered and mixed with the lake water in the ratio of 30:70. Zebra and carp species of fishes were put in the mixed water and observed. Zebra survived only up to 5 hours because of space restriction. While carp survived over a week. Zebra fishes are sensitive compared to carp. Since the carp fishes survived for long time duration, the dilution ratio selected was found to be reasonable.
Conclusion
Based on the BECC studies on Domestic Wastewater (DWW) and Kitchen Wastewater (KWW), the following conclusions are drawn. BECC results for the above said wastewaters have proved the usefulness and effectiveness of the technology for the removal of COD and TDS to the desired level in a short Electrolysis Time (ET).
The wastewater was subjected to BECC studies for different cell voltages 5 V, 7 V and 10 V using stainless steel, copper and iron electrodes for an electrolysis time of 75 min. BECC using SS was carried out in a 1L ECR for cell voltages of 5 V, 7 V and 10 V with the corresponding current density of 0.05, 0.08, 0.1 A respectively. The results show that the maximum removal of COD from 800 mgL1 to 96 mgL-1. The removal efficiency of 88% of COD and 60.41% of TDS were observed at 75min ET for the cell voltage of 10 V. Noting the performance of SS electrodes, HRT was kept as 60 min for further experiments. A series of BECC experiments were carried out by increasing the electrode pairs by 2, 4, 6 and 8 respectively for a fixed applied potential of 10 V for the corresponding current of 0.2 A. It was observed that on every addition of electrode pair in each experiment, a drop in current was observed across the electrodes during treatment. COD and TDS removal efficiency of 90.91% and 75.51% was achieved using 8SS electrodes respectively.
The BECC experiments were carried out in an ECR of 1 L volume. The operating conditions were 4E: SS, Cu and Fe; electrode spacing of 1 cm; agitation speed of 350 rpm; cell voltages: 8, 12 and 16 V; ET: 75 min; COD0: 1120 mgL-1 to 1920 mgL-1; TDS0: 1880 mgL-1 to 2080 mgL1 and pHo: 5 to 6.12. The initial COD value of raw wastewater was 1120 mgL-1; COD gradually decreased to 160 mgL-1 with COD removal efficiencies of 85.71% at the end of 75 min for the cell voltage of 16 V. TDS reduction was maximum at 12 V with the removal efficiency of 42.3%. A series of BECC experiments were carried out by increasing the electrode pairs by 2, 4, 6 and 8 respectively for a fixed applied potential of 16 V with the corresponding current densities of 1.3 A, 0.4 A, 0.3 A & 0.2 A. The experiments were carried out under the operating conditions of ECR: 1 L; ET: 75min; electrode spacing: 1cm; agitation speed: 350 rpm. COD removal efficiency is higher in lesser number of electrodes. As the number of electrodes increases current gets distributed. Using 2SS COD removal efficiency of 87.5% was observed. While using 6SS, TDS removal efficiency of 51.92% was achieved at 16V after ET of 75 min. For an applied current of 0.4 A BECC were conducted using 2, 4, 6 and 8 pairs of electrodes. 85.71% of COD removal was achieved using 2SS electrodes. 50% of TDS removal was observed while using 6SS electrodes. BECC experiments were carried out for discrete ETs for KWW using SS electrodes for applied cell voltage of 16 V. The initial COD value of raw KWW was 1920 mgL-1. COD was reduced to 320 mgL-1 with the removal efficiency of 83.33% and 68.08% of TDS reduction was observed at 75 min ET.
BECC for KWW was conducted using SS electrodes with a fixed applied cell voltage of 16V for 75min ET. Post ECC supernatant was filtered and mixed with the lake water in the ratio of 30:70. Zebra and carp species of fishes were put in the mixed water and observed. Zebra survived only up to 5 hours because of space restriction. While carp survived over a week. Zebra fishes are sensitive compared to carp. Since the carp fishes survived for long time duration, the dilution ratio selected was found to be reasonable.
References
- Karichappan T, Venkatachalam S, Jeganathan PM. Optimization of electrocoagulation process to treat grey wastewater in batch mode using response surface methodology. J Environ Health Sci Eng. 2014;12:29.
[Crossref] [Google Scholar] [Indexed]
- Karabelnik K, Koiv M, Kasak K, et al. High-strength greywater in compact hybrid filter systems with alternative substrates. J Ecol Eng. 2012;49:84-92.
- Katukiza AY, Ronteltap M, Niwagaba CB, et al. A two-step crushed lava rock filter unit for greywater treatment at household level in an urban slum. J Environ Manage. 2014;133:258-267.
[Crossref] [Google Scholar] [Indexed]
- Barisci S, Turkay O. Domestic greywater treatment by electrocoagulation using hybrid electrode combinations. J Jwpe. 2016;10:56-66.
- Bukhari AA. Investigation of the electro-coagulation treatment process for the removal of total suspended solids and turbidity from municipal wastewater. J Bior Tech. 2008;99:914-21.
[Crossref] [Google Scholar] [Indexed]
- Chin WH, Roddick FA, Harris JL. Greywater treatment by UVC/H2O2. Water Res. 2009;43(16):3940-3947.
[Crossref] [Google Scholar] [Indexed]
- Impa JA, Nagarajappa DP, Gowda KK, et al. Domestic wastewater treatment by electrocoagulation using copper and aluminum electrodes. Intr J Innovative Research in Science, Engineering and 2015;4(6):3844-50.
- Janpoor F, Torabian A and Khatibikamal V. Treatment of laundry waste-water by electrocoagulation. J Chem Technol Biotechnol. 2011;86(3):1113–1120.
- Nguyen DD, Kim SD, Yoon YS. Enhanced phosphorus and COD removals for retrofit of existing sewage treatment by electrocoagulation process with cylindrical aluminum electrodes. J Desalination and Water Treatment. 2014;52:2388–2399.
- Sharma AK and Chopra AK. Removal of COD and BOD from biologically treated municipal wastewater by electrochemical treatment. J Applied and Natural Sciences. 2013;5(2):475-481.
- Shreesadh EC, Thakur S, Chauhan MS. Electro-coagulation in wastewater treat Intr J Engineering and Science Research. 2014;4:584-89.
- Vakil KA, Sharma MK, Bhatia A, et al. Characterization of greywater in an Indian middle-class household and investigation of physicochemical treatment using electrocoagulation. J Separation and Purification Technology. 201; 130: 160-66. .
- Wang C T, Chou W L, Kuo Y M. Removal of COD from laundry wastewater by electrocoagulation/electro flotation. J Hazardous Materials. 2009;164:81-86.
[Crossref] [Google Scholar] [Indexed]
- Wu S, Kuschk P, Brix H, et al. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. J Water Research. 2014;57:40-55.
[Crossref] [Google Scholar] [Indexed]
- Yadav AK , Singh L , Mohanty A , et al. Removal of various pollutants from wastewater by electrocoagulation using iron and aluminium electrode. J Desalination and Water Technology. 2012;46:352-58.