Original Article
, Volume: 16( 1)An Efficient and Stereoselective Synthesis of S-(+)-Pregabalin Using a Chiral Auxiliary Reagent
- *Correspondence:
- Mangatayaru KG Department of Chemistry, Palamuru University, Mahabubnagar, Telangana, India, Tel: +91-9985362601; E-mail: kotugirijamangatayaru@gmail.com
Received Date: December 19, 2017 Accepted Date: January 28, 2018 Published Date: February 03, 2018
Citation: Vinigari K, Murugan RN, Noorjahan M, et al. An Efficient and Stereoselective Synthesis of S-(+)-Pregabalin Using a Chiral Auxiliary Reagent. Int J Chem Sci. 2018;16(1):246
Abstract
A highly efficient and enantio selective synthesis of (S)-3-amino-5-methylhexanoic acid 1 (Pregabalin) is reported. Evans enolate based asymmetric alkylation reaction was used as the key step to set up the required chirality. This procedure allows the rapid process to provide the (S)-(+)-Pregabalin 1 in excellent overall yields and purity.
Keywords
Pregabalin; Alkylation; Enantiomers; Enolate; Oxazolidinone; Retrosynthesis
Introduction
Pregabalin 1 ((S)-(+)-3-amino methylhexanoic acid) is a novel and potent anticonvulsant agent for the treatment of epilepsy and pain [1]. It has also been found to be more active than Gabapentin in preclinical models of epilepsy [2]. Although the mixture of enantiomers did not possess the pharmacological activity in clinical trials, [3] the S enantiomer of pregabalin possesses the required biological activity. Hence the stereo selective synthesis of (S)-(+)-Pregabalin is of great interest to the research scientists.
The first synthesis of (S)-(+)-Pregabalin was reported by Hoekstra et al. 1a in the Warner-Lambert Laboratory with several novel manufacturing processes [3]. Most of the synthesis of (S)-pregabalin involves the use of asymmetric hydrogenation [4] or use of chiral resolving agent [5]. However recently sartillo et al. [6] have reported the chiral auxiliary based radical cyclization to get the (S)-pregabalin. In this paper, we report our strategy involving chiral auxiliary based Evans asymmetric alkylation approach for the synthesis of the (S)-(+)-Pregabalin with high optical activity.
Experimental
The reactions were conducted in over dried glass ware with mechanical stirrer. Chemicals were parched from local venders. 1H NMR spectra were recorded on a Gemini-2000 (200 MHz) and Mercury plus (varian 400 MHz) spectrometer with CDCl3 and D2O as the solvent. Mass spectra were recorded on Xevo TQD Triple Quadrupole Mass Spectrometry and the FT-IR measured on a Perkin Elmer spectrum 100. The specific optical rotation of pregabalin was carried out on modal P-1030 Polari meter.
Preparation of compound 1: In an autoclave vessel, added 10% Pd/C (3 g, 10% load) to the solution of compound 9 (30 gm, 0.11 mol) in ethyl acetate in the presence of hydrogen atmosphere (3-4 Kg/cm2) for 16 hours at 25-30°C. Pd/C catalyst was filtered off and the filtrate was concentrated under vacuum. Next, water (8 mL) and isopropanol (150 mL) were added to the above concentrated solution. Then, the temperature was raised to 60°C, maintained for 40 minutes and allowed to cool slowly and maintained at 0-5°C for 2-3 hours. Filtered the isolated solid and washed with chilled isopropanol (40 mL) and dried at 45-50°C to give the pure compound 10 with 70% yield (12.14 g).
Compound 1: [α]D25.7+10.84 (c 1, H2O). 1H NMR (200 MHz, CDCl3)  ppm: 0.86-0.87 (3H, d, J=1.9 Hz), 0.89-0.91 (3H, d, J=1.9 Hz), 1.18-1.24 (2H, m), 1.58-1.75 (1H, m), 2.12-2.45 (3H, m), 2.94-3.10 (2H, m). IR (KBr, cm-1): 3435, 2893, 1644, 1551, 1390, 1279, and 702. Mass: 160.1 (M++1). Anal. Calcd for C8H17NO2.
ppm: 0.86-0.87 (3H, d, J=1.9 Hz), 0.89-0.91 (3H, d, J=1.9 Hz), 1.18-1.24 (2H, m), 1.58-1.75 (1H, m), 2.12-2.45 (3H, m), 2.94-3.10 (2H, m). IR (KBr, cm-1): 3435, 2893, 1644, 1551, 1390, 1279, and 702. Mass: 160.1 (M++1). Anal. Calcd for C8H17NO2.
Results and Discussion
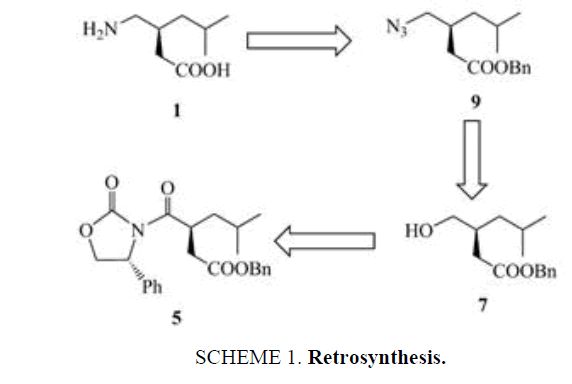
The retro synthetic analysis for our planned synthesis of S-(+)-Pregabalin 1 is shown in Scheme 1. The azido ester 9 the precursor to the pragabalin could be obtained from the hydroxy ester 7, which in turn was obtained from the oxazolidinone 5.
As outlined in Scheme 2, the coupling of the chiral oxazolidinone auxiliary 3 with the acid 2 was achieved by using the mixed anhydride method [7]. The acid was first activated with Pivoloyl chloride by forming the mixed anhydride. Chemo selective attack at the less hindered carbonyl group by the chiral auxiliary 3 led to the desired product. This protocol avoids the need for isolating the unstable, corrosive acyl chloride and seems to offer advantages in terms of practicality. Now the key step in our synthesis is the alkylation of the lithium enolate of 4 with benzyl bromo acetate afforded the alkylated product 5 with excellent diastereo selectivity. The desired diastereomer was isolated in 80% of yield and with >99.0% d.e after recrystallization from tert-butyl methyl ester.
Scheme 2: Reagents and conditions: (a) Pivoloyl Chloride, CH2Cl2, Et3N, RT to reflux, 6-8 h, 80%; (b) LiHMDS, Benzyl bromo acetate, -78°C, 2 h, 85%; (c) LiOH, H2O2, THF:H2O, -5°C, 3 h, 85%; (d) BH3.Me2S, THF, 0°C, 4 h, 84%; (e) CH3SO2Cl, CH2Cl2, Et3N, DMAP, 0°C RT, 2 h, 90%; (f) NaN3, DMF, 90°C, 6 h, 75%; (g) 10% Pd/C, H2, 20 Psi, Methyl t-Butyl Ether, RT, 6 h, 78%.
Removal of the chiral auxiliary was achieved by selective hydrolysis of amide using Lithium peroxide to produce the corresponding acid 6 without racemization of the newly formed stereogenic center [8]. The chiral auxiliary was readily separated from the product by simple acid/base extraction.
The carboxylic acid 6 was selectively reduced to the corresponding alcohol 7 by using Borane Dimethylsulphide [9]. Mesylation of the alcohol followed by displacement of mesylate with sodium azide gave the azido ester 9 [10]. Finally, in situ removal of the benzyl ester group and reduction of azide to amine was achieved by using 10% Pd/C in presence of H2 at room temperature to give the enantiomerically pure (S)-(+)-Pregabalin 1. [α] D+10.84 (c 1.0, H2O) (Lit. [α] D+10.1 (c 1.1 in H2O]. The physical and spectroscopic data of all the compounds are in good agreement with the proposed structures and those of 1 and 9 are in good agreement with the literature data [11].
Conclusion
In conclusion, we have demonstrated a seven step synthesis of S-(+)-Pregabalin by using Evan’s oxazolidinone based alkylation as the key step. The present synthesis is having an overall yield of 25% and provides easy accesses for the synthesis in S-(+)-Pregabalin in kilogram scale. The synthesis of other biologically active compounds by using chiral auxiliary based strategy is being investigated in our laboratory.
Acknowledgement
We thank the management of Dr. Reddy’s Laboratories Ltd. for extending support to the work. Co-operation from the project colleagues and analytical department is highly appreciated.
References
- (a) Yuen PW, Kanter GD, Taylor CP, et al. Enantioselective synthesis of PD144723: A potent stereospecific anticonvulsant. Bioorg & Med Chem Lett. 1994;4(6):823-6.
(b) Bryans JS, Davies N, Gee NS, et al. Identification of novel ligands for the gabapentin binding site on the α2δ subunit of a calcium channel and their evaluation as anticonvulsant agents. J Med Chem. 1998;41:1838-45.
(c) Butters M, Catterick D, Craig A, et al. Critical assessment of pharmaceutical processes: A rationale for changing the synthetic route. Chem Rev. 2006;106(7):3002-27. - (a) Belliotti TR, Capiris T, Ekhato IV, et al. Structure activity relationships of pregabalin and analogues that target the α2-δ protein. Journal Med Chem. 2005;48(7):2294-307.
(b) Taylor CP. In: New Trends in Epilepsy Management. Chadwick D, editor. Royal Society of Medicine Services Ltd: London. 1993;pp:13-40. - Hoekstra MS, Sobieray DM, Schwindt MA, et al. Chemical development of CI-1008, an enantiomerically pure anticonvulsant. Org Proc Res & Dev. 1997;1(1):26-38.
- (a) Burk MJ, de Koning PD, Grote TM, et al. An enantioselective synthesis of (S)-(+)-3-aminomethyl-5-methylhexanoic acid via asymmetric hydrogenation. J Org Chem. 2003;68(14):5731-4.
- Ordo´nez M, Cativiela C. Stereoselective synthesis of γ-amino acids. Tetrahedron: Asymmetry 2007;18:3-99.
- Rodriguez V, Quintero L, Sartillo-Piscil F. Stereoselective 5-exo-trig radical cyclization in the enantioselective synthesis of Pregabalin. Tetrahedron Letters. 2007;48(24):4305-8.
- (a) Prashad M, Kim HY, Lu Y, et al. The first enantioselective synthesis of (2 R, 2 ‘R)-threo-(+)-methylphenidate hydrochloride. J Org Chem. 1999;64:1750-3.
(b) Evans DA, Ennis MD, Mathre DJ. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of alpha-substituted carboxylic acid derivatives. J Am Chem Soc. 1982;104(6):1737-9. (c) Wittenberger SJ, McLaughlin MA. Preparation of endothelin antagonist ABT-627. Tetrahedron Lett. 1999;40:7175-78.
(d) Hilpert H. Practical approaches to the matrix metalloproteinase inhibitor Trocade® (Ro 32-3555) and to the TNF-α converting enzyme inhibitor Ro 32-7315. Tetrahedron. 2001;57(36):7675-83. - Haigh D, Birrell HC, Cantello BC, et al. Non-thiazolidinedione antihyperglycaemic agents. Part 5: Asymmetric aldol synthesis of (S)-(−)-2-oxy-3-arylpropanoic acids. Tetrahedron: Asymmetry. 1999;10(7):1353-67.
- Pelter A, Smith K, Brown HC. Borane Reagents, Academic; London. 1989.
- Chavan SP, Praveen C. A concise and stereoselective synthesis of (+)-and (−)-deoxoprosophylline. Tetrahedron Letters. 2004;45(2):421-3.
- Taedong OK, Aram J, Joohee L, et al. A key intermediate to concise synthesis of pregabalin. J Org Chem. 2007; 72(19):7390-3.