Original Article
, Volume: 12( 11)Allelopathic Effects of Aqueous Extract of Lantana camara L. on Seed Germination of Black Gram Vigna mungo L.
- *Correspondence:
- Nadirsha PS Nawab , Msc, Department of Ecology and Environmental Sciences, Pondicherry University,Pondicherry, India, Tel: +919497309720; E-mail: nadirshapsnawab@gmail.com
Received: October 15, 2016; Accepted: November 24, 2016; Published: November 30, 2016
Citation: Nadirsha PS Nawab, Yogamoorthi A. Allelopathic Effects of Aqueous Extract of Lantana camara L. on Seed Germination of Black Gram Vigna mungo L. Environ Sci Ind J. 2016;12(11):122.
Abstract
An attempt was made to assess the allelopathic effects of aqueous extract of Lantana camara L. a potential invasive plant on growth and seedling related traits of black gram Vigna mungo L. The seeds were treated in 10%, 20%, 40%, 80% and 100% concentrations. The leaf aqueous extract of Lantana camara L. showed significant inhibitory activity on germination potency of the seed in terms of Germination Index, Tolerance Index, and seed coat shedding time. Besides, Phytotoxicity, for the Vigna mungo L. were found out. Germination Index and Tolerance Index were found to be decreased with increase in the concentration of aqueous leaf extracts and phytotoxicity increased. The maximum and minimum inhibitory values were obtained in 100% and 10% extract concentrations respectively. An attempt was also made to estimate the enzymatic activity. All the results and observations obtained in this experimental study were discussed in the view of phyto-chemical constituents of Lantana camara L. and their probable allelopathic property against the seed germination.
Keywords
Allelopathic; Lantana camara L.; Seed germination; Vigna mungo L.; Germination index
Introduction
Allelopathy is an interesting but complex mode of interaction between plants, accomplished through the release of chemical substances into the environment. Different groups of plants like algae, lichens, annual and perennial weeds have wide known allelopathic interactions, [1]. The donor plants may affect germination, growth, and development of the recipient plants species [2]. Lantana camara L. belongs to the family Verbenacea is one of the known weed plants in the many parts of the world especially in tropics and it has been widely evaluated for its allelopathic property against important weeds and crops. Ahmad et al. [3] evaluated the allelopathic effects of Lantana camara L. aqueous extract on seed germination and seedling growth of some agricultural crops followed by Enyew et al. [4] evaluated in Maize, Zeamays, Wheat, Triticum turgidum L.; Pogonatu maloides L.; El-Kenany et al. [5] studied on the germination of Phalaris minor L.; Ranwala et al. [6] evaluated in Ludwigia sp L.; Bansal et al. [7] in rice; Gantayet et al. [8] in Green gram; Gentle et al. [9] on Australian forest species; Hossain et al. [10] in agricultural crops of Bangladesh; Binggeli et al. [11] in drive peoples out of their lands; Oudhiya et al. [12] studied in soya bean; Saxena et al. [13] in Water hyanth; Mishra et al. [14] in Trigonella; Rugare et al. [15] in Black jack; Tadele et al. [16] studies the allelopathic effects in agricultural crops; Kong et al. [17] in Eichornnia; Kumbhar et al. [18] on crop plants; Achhireddy et al. [19] in Morrenia odorata L.; Dobhal et al. [20] in woody shrubs. Besides Lantana Camara L. other plants also tested for understanding the possible physiological mechanisms behind the allelopathic effects on black Gram; Suman et al. [21]; Siddiqui et al. [22] in Black pepper leaching; Singh et al. [23] tested in leaf leachate of eucalyptus; Shankar et al. [24] evaluated allelopathic effects of phenolics and terpenoids extracted from Gmelina arborea on germination of Black gram; Effect of salicylic acid on nodulation, nitrogenous compounds and related enzymes of Vigna mungo L. However, experimental studies reported that the mechanisms involved in tolerance of crop seedlings subjected to stressed environment Prasad et al. [25]; Palma et al. [26] reported the role of peroxisomal proteases in different physiological events that take place under stress situations is discussed followed by Foyer et al. [27] studied important defense mechanism in transgenic plants; Roy?Macauley et al. [28] evaluated proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. In the present study, an experiment was conducted to assess the allelopathic effect of aqueous extract of Lantana camara L. extract on black gram Vigna mungo L. as it has not been tested previously.
Materials and Methods
Materials
Collection of Lantana camara L. plant materials: Fresh and healthy leaves were collected from garden land of Pondicherry University Experimental Farm Figure 1. The leaf washed in running tap water for removing the surface contaminants and dust and dried at room temperature. The plant materials were chopped into small pieces with cutter [29].
Collection of Vigna mungo L. seeds: Well mature Vigna mungo L. seed were collected from the Pondicherry Agro Service and Industries Corporation (PASIC) in the first week of July 2015. The seed kept under sunlight for 3 h. Then the seed were soaked with Sodium Hypochlorite solution in 5 min for Sterilization [30].
Preparation of Lantana camara L. aqueous extracts (LCAE): The Leaf was soaked in distilled water for one hour at room temperature. The extract obtained after the Grinding of leaf pestle and motor in the ratio of 30 g leaf: 100 ml distilled water. The crude extract is boiled at 60°C for 30 min and the crude extract is centrifuged at 5000 rpm for 10 min, supernatant obtained after the centrifugation is filter through a Whatman No. 1 filter paper and diluted the filtrate with distilled water to prepare different concentrations (10%, 20%, 40%, 80%, and 100%) according to the treatments [31].
Methods
Experimental setup: Petri plates were given a thorough washing with detergent using hot water as precautionary measure against pathogens and pollutants. Ten seeds of Vigna mungo L. were sown in each Petri plates of a 9-cm diameter. 6 ml of solution was applied to Petri plates and control treatment received 6 ml of distilled water. Both treated and control Petri plates were kept moist continuously by adding respective concentrations in duplicate. The Petri plates were kept at room temperature (30 ± 4)°C throughout the study. Germination counts were recorded at the end of each day for 3 days continuously. Morphological feature’s viz, Radicle length, timing of seed coat shedding, nature of the seed were recorded each day. The experiment was laid out in complete randomized block design with 3 replicates (Figure. 2-Figure 4).
Estimation of enzyme activity
Estimation of protease: The protease content in seed was estimated by Ladd and Butler method [32]. Place 1 ml of sample in a 250- ml conical flask. Add 5 ml Triss buffer and 5 ml of sodium caseinate solution. Stopper the tubes, mix the content and incubator for 2 h at 50°C on a shaking water bath. At the end of incubation add 5 ml of trychloroacetic acid (TCA) solution and mix the content thoroughly. To perform the control, add 5 ml of sodium caseinate solution at the end of the incubation and immediately before adding the TCA solution. Centrifuge the sample at 10,000 rpm for 10 min. Pipette 5 ml of the clear supernatant into tubes mix with 7.5 ml of alkaline reagent, and incubate for 15 min at room temperature. After add 5 ml of the follin reagent, filter the mix through paper filter into glass tube and measure the absorbance after 1 h at 700 nm.
Evaluation Index
Germination index: Germination/emergence index (GI/EI) was calculated by following formula used by Association of Official Seed Analysis (AOSA, 1978).
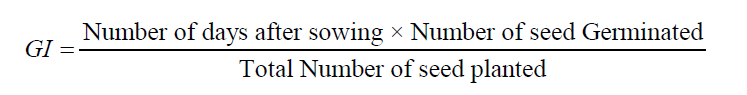
Tolerance index: Tolerance index was calculated by using the formula suggested by Turner and Marshal (1972). Longest root in treatment of the seedling was measured for the calculation of Tolerance Index (TI) as follows:
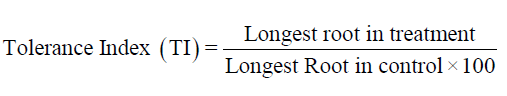
Percentage of toxicity: The percentage of the toxicity of the seeds was calculated by the formula suggested by Chiou and Muller [33] as follows.
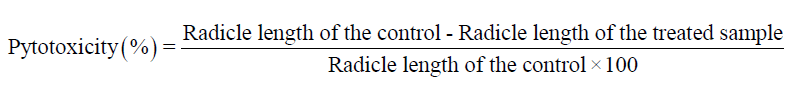
Results
The results obtained from the present study showed that, seed treated with aqueous extract of the donor plant will have more inhibitory effect on recipient plant in terms of germination percentage, Tolerance Index, phytotoxicity, shedding of seed coat, and it was given in Table 1. The highest concentration (100%) of aqueous leaf extracts showed maximum inhibitory effects in terms of germination percentage, root length when compared to control. The rate of inhibition increased with increasing extract concentration. The length of the radicle in control and 100% (Table 1) were found to be increased each day of experiment. The maximum radicle length of were measured in 3rd day of experiment, but compare to control the length of the radicle reduced in 100% of extract concentration. Similarly, radicle length of seed soaked in 20% and 40% concentration were found to be increased in each day. The maximum radicle length observed in 3rd day of treatment in 20% and shortened in 40% of concentration.
| No of Seeds | Control (Distilled water) | 100% of leaf Extracts | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st day | 2nd day | 3rd day | 1st day | 2nd day | 3rd day | |||
| 1 | 0.8 | 1.4 | 1.9 | 0.2 | 0.2 | 0.2 | ||
| 2 | 0.8 | 1.5 | 2.0 | 0.3 | 0.3 | 0.3 | ||
| 3 | 0.7 | 1.4 | 1.7 | 0.3 | 0.3 | 0.3 | ||
| 4 | 0.9 | 1.5 | 1.8 | 0.4 | 0.4 | 0.4 | ||
| 5 | 0.8 | 1.6 | 1.9 | 0.5 | 0.5 | 0.5 | ||
| 6 | 0.8 | 1.4 | 1.8 | 0.3 | 0.3 | 0.3 | ||
| 7 | 0.7 | 1.6 | 1.9 | 0.4 | 0.4 | 0.4 | ||
| 8 | 0.8 | 1.3 | 1.8 | 0.5 | 0.5 | 0.5 | ||
| 9 | 0.9 | 1.3 | 2.1 | 0.3 | 0.3 | 0.3 | ||
| 10 | 0.9 | 1.5 | 1.9 | 0.3 | 0.3 | 0.3 | ||
| Mean | 0.81 | 1.45 | 1.88 | 0.35 | 0.35 | 0.35 | ||
| SD | 0.07379 | 0.10801 | 0.11353 | 0.097 | 0.097 | 0.097 | ||
| Germination Index | 3 | 3 | 3 | 0.9 | 0.9 | 0.9 | ||
Table 1: Measurement value of radicle length, germination index, phytotoxicity and tolerance index in each day of experiment (cm) of maximum (100%) and minimum concentrations (control).
The length of the radicle of seed soaked in 80% and 100% concentrations were found to be reduced. When compare to all other concentration 80% and 100%, the length of the radicle found to be decreased. The maximum reduction of radicle length found in 100% concentration. Germination index also was significantly affected by two concentrations 80% and 100%. The tolerance index and phytotoxicity of seed soaked in Control and 100% is represented in Table 1 From the Experimental result shows that growth parameters i.e., Germination Index, Tolerance Index of the seed found to be reduced and phytotoxicity increased with increasing the concentration of Leaf extracts. Shedding of the seed were decreases with increasing the concentration.
The maximum seed shedding was observed in control and minimum at 100% concentration. Other observed morphological changes include inhibited or retarded germination rate; seeds darkened and swollen; reduced radicle and swelling or necrosis of root tips; curling of the root axis; discoloration, lack of root hairs; increased number of seminal roots.
Vigna mungo L. seed exhibit increasing trends in protease activity in all the three concentration from the period of 24 h of germination, and it continued until 72 h of seed germination. The maximum protease activity was found in 72 h of the germination and minimum found in 24 h of the seed germination. The maximum protease activity was found in seed treated with 100% concentration of the Lantana camara L. extract 7.987 ppm and minimum was found in control 2.245 ppm given in Tables 2 and 3.
| Day 1 | Day 2 | Day 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations | 10% | 20% | 40% | 80% | 100% | 10% | 20% | 40% | 80% | 100% | 10% | 20% | 40% | 80% | 100% |
| Phytotoxicity μmol. L | 7.40 | 12.40 | 25.3 | 49.38 | 56.79 | 37.2 | 38.6 | 51.0 | 71.3 | 75.8 | 16.4 | 33.5 | 62.7 | 77.1 | 81.32 |
| Tolerance index | 155.5 | 133.3 | 111 | 77.7 | 55.5 | 78.75 | 68.75 | 50 | 25 | 15 | 90.4 | 66.6 | 47.6 | 33.2 | 23.80 |
Table 2: Trends in phytotoxicity and tolerance index in various concentrations of 3 days of experiment.
| Enzymes | Protease in PPM | ||
|---|---|---|---|
| Days | Control | 50% | 100% |
| D1 | 2.247 ppm | 2.36 ppm | 3.985 ppm |
| D2 | 2.745 ppm | 4.187 ppm | 5.145 ppm |
| D3 | 3.643 ppm | 5.248 ppm | 7.987 ppm |
Table 3: Effect of aqueous extracts on enzyme activity.
Discussion
Allelopathy is an interesting but complex mode of interaction between plants, accomplished through the release of chemical substances into the environment. Different groups of plants like algae, lichens, annual and perennial weeds have wide known allelopathic interactions.
The present study envisages that aqueous extracts of Lantana camara L. (donor species) exhibit strong inhibitory allelopathic effect on the germination process of Vigna mungo L. The germination was highly sensitive to the donor aqueous extract in various treatments. According to Cruz-ortega et al. [34] inhibition of root length may be due to the presence of phenols. It is possible that these phenolic compounds interfered with the phosphorylation pathway and inhibiting the activation of Mg2+ and ATP–ase activity. Verdeguer et al. [35] studied the Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops and he found that the reduction of root and shoot radicle is due to aromatic alkaloids and phenolic compounds which interfere with germination and growth of species; many different secondary metabolitese. g., phenolics, terpenoids, alkaloids, polyacetylenes, fatty acids, and steroids have ability to retard the growth of other plants by interfering with metabolic activity [36]; the presence of 14 type of allelochemicals present in plant mainly Lantadene A (Penta cyclic triterterpinoids) and Lantadene B will alter the energy circulation and the enzyme, protein and amino acid activity during the period of germination of recipient plants [37]. Our result obtained from the analysis of enzyme showed that increasing trends in protease activity. However Solomon et al. [38]; Ryan et al. [39]; Hatsugai et al. [40]; Kim [41]; Dubey et al. [42]; Silveira et al. [43]; Sweetlove et al. [44]; reported that protease have ability to respond stressed environment through synthesis of bio molecules and this enzyme will act as a plant defense against toxic chemicals. Thus, our results suggested that leaf aqueous extract having a potential allelopathic effect. Meanwhile, further studies under field conditions are necessary to evaluate the possible use of L. camara aqueous extract or as bio herbicides against certain weeds that resistant to available pesticides.
References
- Rice EL. Allelopathy. Cambridge: Academic Press, England; 2013. 366 p.
- Einhellig FA. Allelopathy: Current status and future goals. In: Inderjit A, Dakshini KMM, editors. Allelopathy: Organisms, processes, and applications. Washington: American Chemical Society, USA; 1995. p 1?24.
- Ahmed R, Arfin-Khan MAS, Sharif AM, et al. Allelopathic effects of Lantana camara on germination and growth behaviour of some agricultural crops in Bangladesh. J For Res. 2007;18(4):301-4.
- Molla AE, Nagappan R. Allelopathic effect of Lantana camara L. Leaf powder on germination and growth behaviour of maize Zea mays Linn. and wheat Triticum turgidum Linn. Cultivars. Asian J Agric Sci. 2015;7(1):4-10.
- Kenany E, Eman T, Salama M. Suppression effects of Lantana camara L. aqueous extracts on germination efficiency of Phalaris minor Retz. and Sorghum bicolor L.(Moench). Journal of Taibah University for Science. 2013;7(2):64-71.
- Sudheera R, Attigala LR, Silva S. Herbicidal potential of Lantana camara l. On Ludwigia spp. in paddy soil. Tropical Agricultural Research and Extension. 2015;17(1):10-18.
- Bansal GL. Allelopathic effect of Lantana camara on rice and associated weeds under the mid-hill conditions of Himachal Pradesh, India. Proceedings of the Workshop on Allelopathy in Rice. Manila (Philippines): International Rice Research Institute. 1998.
- Gantayet PK, Adhikary SP, Lenkaet KC, et al. Allelopathic impact of Lantana camara on vegetative growth and yield components of green gram (Phaseolus radiatus). Int J Curr Microbiol App Sci. 2014;3(7):327-35.
- Gentle CB, Duggin JA. Allelopathy as a competitive strategy in persistent thickets of Lantana camara L. in three Australian forest communities. Plant Ecology. 1997;132(1):85-95.
- Kamal HM, Alam N. Allelopathic effects of Lantana camara leaf extract on germination and growth behavior of some agricultural and forest crops in Bangladesh. Pakistan Journal of Weed Science Research. 2010;16(2):217-26.
- Pierre B, Desissa D. Lantana camara-the invasive shrub that threatens to drive people out of their land. Newsletter of the Ethiopian Wildlife and Natural History Society. 2002.
- Oudhia, P. Allelopathic effects of Lantana camara L. On germination of soyabean. Legume Research. 1999;22:273-4.
- Saxena MK. Aqueous leachate of Lantana camara kills water hyacinth. J Chem Ecol. 2000;26(10):2435-47.
- Arpana M. Phytotoxic effect of Lantana camara leaf extract on germination and growth behavior of Trigonella foenum-graceum L. International Journal of Scientific Research. 2013;2(5):18-9.
- Rugare JT. Allelopathic effects of Lantana (Lantana camara) on blackjack (Bidens pilosa) and pearl millet (Pennisetum glaucum). Asian Journal of Agriculture and Rural Development. 2013;3(8):543-53.
- Tadele D. Allelopathic effects of Lantana (Lantana camara L.) Leaf extracts on germination and early growth of three agricultural crops in Ethiopia. Momona Ethiopian Journal of Science. 2014;6(1):111-9.
- Kong CH, Wang P, Zhang CX, et al. Herbicidal potential of allelochemicals from Lantana camara against Eichhornia crassipes and the alga Microcystis aeruginosa. Weed research. 2006;46(4):290-5.
- Kumbhar BA, Patel GR. Phytotoxic effects of Lantana on hypocotyl and radicle growth of some crops of Patan. Int J Int sci Inn Tech Sec C. 2013;2(6):8-11.
- Achhireddy NR, Megh S. Allelopathic effects of Lantana (Lantana camara) on milkweed vine (Morrenia odorata). Weed Science. 1984;32(6):757-61.
- Dobhal PK, Kohli RK, Batish DR. Evaluation of the impact of Lantana camara L. Invasion, on four major woody shrubs, along Nayar river of Pauri Garhwal, in Uttarakhand Himalaya. Int J Biodivers Conserv. 2010;2(7):155-61.
- Archna S, Shahi HN, Pushpa S, et al. Allelopathic influence of Vigna mungo (black gram) seeds on germination and radical growth of some crop plants. Plant Growth Regulation. 2002;38(1):69-74.
- Siddiqui ZS. Allelopathic effects of black pepper leachings on Vigna mungo (L.) Hepper. Acta Physiol Plant. 2007;29(4):303-8.
- Singh NB, Singh R. Effect of leaf leachate of eucalyptus on germination, growth and metabolism of greengram, blackgram and peanut. Allelopathy Journal. 2003;11(1):43-51.
- Shankar SRM, Girish R, Karthik N, et al. Allelopathic effects of phenolics and terpenoids extracted from Gmelina arborea on germination of Black gram (Vigna mungo) and Green gram (Vigna radiata). Allelopathy Journal. 2009;23(2):323-32.
- Prasad TK. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: Changes in antioxidant system, oxidation of proteins and lipids, and protease activities. The Plant Journal. 1996;10(6):1017-26.
- Palma JM, Sandalio LM, Corpas FJ, et al. Plant proteases, protein degradation, and oxidative stress: Role of peroxisomes. Plant Physiology and Biochemistry. 2002;40(6):521-30.
- Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant, Cell & Environment. 1994;17(5):507-23.
- Macauley RH, Fodil YZ, Kidric M, et al. Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiologia Plantarum. 1992;85(1):90-6.
- El-Lakwah FA, Hamed MS, Abdel-Latif AM. Effectiveness of Lantana camera and Nerium oleander extracts alone and in mixtures with two insecticides against Rhizopertha dominica (F.). Annals of Agricultural Science Moshtohor. 1996;34(4):1879-1905.
- Sahid IB, John BS. Allelopathic effect of Lantana (Lantana camara) and Siam weed (Chromolaena odorata) on selected crops. Weed science. 1993;41(2):303-8.
- Rajbanshi SS, Inubushi K. Chemical and biochemical changes during laboratory-scale composting of allelopathic plant leaves (Eupatorium adenophorum and Lantana camara). Biol Fertil Soils. 1997;26(1):66-71.
- Ladd JN, Butler JHA. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol Biochem. 1972;4(1):19-30.
- Chiou CH, Muller CH. Allelopathic mechanism of Archtostaphylous glandulosa variety Zazaeisis, American Mediterranean. Nature. 1972;88:324-47.
- Cruz OR, Gabriela AC, Ana LA. Allelochemical stress produced by the aqueous leachate of Callicarpa acuminata: effects on roots of bean, maize, and tomato. Physiologia Plantarum. 2002;116(1):20-7.
- Verdeguer M, Blázquez M, Herminio B. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem Sys Ecol. 2009;37(4):362-9.
- Inderjit A. Plant phenolics in allelopathy. The Botanical Review. 1996;62(2):186-202.
- Sharma OP, Dawra RK, Makkar HPS. Isolation and partial purification of Lantana (Lantana camara L.) toxins. Toxicol Lett. 1987;37(2):165-72.
- Solomon M, Beatrice B, Massimo D, et al. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. The Plant Cell. 1999;11(3):431-43.
- Ryan CA. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu Rev Phytopathol. 1990;28(1):425-49.
- Hatsugai N, Miwa K, Kenji Y, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305(5685):855-8.
- Kim JY, Seong CP, Indeok H, et al. Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci. 2009;10(6):2860-72.
- Dubey RS. Protein synthesis by plants under stressful conditions. In: Pessarakli M, editor. Handbook of Plant and Crop Stress 2. New York City: Marcel Dekker, USA; 1999. p 365-97.
- Silveira JA, Viégas RA, Da Rocha IM, et al. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J Plant Physiol. 2003;160(2):115-23.
- Sweetlove LJ, Heazlewood JL, Herald V, et al. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32(6):891-904.





