Original Article
, Volume: 12( 1)Alkali Roasting of Titania Slag for Preparation of High Grade -TiO2
- *Correspondence:
- Salah A Nuclear Materials Authority, El-Maadi, Cairo, Egypt, Tel: +20227572499; E-mail: salah.afify@yahoo.com
Received: December 21, 2016 Accepted: February 26, 2017 Published: March 03, 2017
Citation: Salah A. Alkali Roasting of Titania Slag for Preparation of High Grade –TiO2. Inorg Chem Ind J. 2017;12(1):106.
Abstract
A high grade TiO2 product of 99.1% purity has successfully been prepared from the Egyptian Rosetta titania slag by its proper roasting with sodium hydroxide. The reaction product was then successively washed by water followed by controlled HCl acid leaching procedures in order not to dissolve the titanium values. The first step has aimed to dissolve silica, alumina besides possible V and Cr as well as recovering the excess NaOH while the HCl acid leaching step was so applied to selectively dissolve the iron and other impurities. The studied optimum conditions of roasting involved a slag/NaOH ratio of 5/6 at a roasting temperature of 850°C for 0.5 h. After both leaching circuits the titanium product left behind was filtered and properly washed with distilled water before drying at 110°C and calcination at 850°C.
Keywords
Titania slag; NaOH; TiO2; HCl
Introduction
Titanium dioxide is actually one of the most commonly required materials in the chemical manufacturing industry; a matter which is due to its wide variety of applications. Middle-mass et al. [1] have reviewed these applications where TiO2 pigment is used in paints, plastics, paper, sunscreen, cosmetics and even as a food additive. Also, TiO2 is used in photovoltaic cells, biomedical devices as well as in air purification.
The two main commercial processes for producing titanium dioxide pigment include actually the sulfate and the chloride processes and their main feedstocks are indeed the high-TiO2 slag and/or synthetic rutile produced from ilmenite ore. In the sulfate process, the feedstock is digested in concentrated sulfuric acid to produce titanium sulfate which is hydrolyzed at boiling temperature after the prior crystallization and separation of iron sulfate. This would be followed by calcination at 650°C to 1000°C to form either anatase or rutile. On the other hand, in the chloride process, the feedstock is reacted with petroleum coke and chlorine gas at high temperatures to give TiCl4 vapor. After distillation of the latter, it was subjected to oxidation at 1300°C to 1800°C to give TiO2 and chlorine gas.
The major disadvantages of the sulfate process is the huge generation of acidic and solid waste besides high energy consumption. Although the chloride process does not produce excessive wastes, it has also several environmental effects which mainly include huge CO2 production. For these reasons, several hydrometallurgical procedures have lately been studied including variable techniques for sulfate treatment followed by solvent extraction in a manner to recycle the raffinate as the lixiviant for the initial leaching step [2]. Similarly Lakshmanan et al. [3] have developed the chloride process by subjecting the leach liquor to solvent extraction for iron removal. On the other hand, Verhulst et al. [4] have developed the Altair process in which the leached iron is mostly removed by crystallization of FeCl2 by proper cooling of the leach liquor which is then subjected to phosphine oxide extraction of both Ti and Fe2+ iron followed by an amine extraction to separate Fe3+ iron. In spite of this progress, these procedures are either consuming large amounts of energy and/or generating large amounts of carbon emissions and solid waste.
In order to overcome these inconveniences, an alternative trend has been investigated involving the alkaline roasting of titanium feedstocks whether using soda ash or sodium hydroxide. In this regard, Foley and Mackinnon [5] have roasted ilmenite concentrates with potassium and sodium carbonate at 860°C, while Elger and Holmes [6] have been able to remove the impurities in the titania slag by roasting with an alkali metal carbonate, sulfate or chloride, while Lahiri and Jha [7] have studied the ilmenite roasting kinetics with soda ash and have indicated that the decomposed products TiO2, FeO and SiO2 would react as follows:
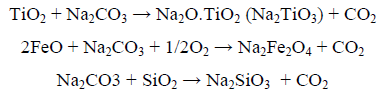
However, in soda ash roasting of Rosetta titania slag, the latter reaction has not taken place as was proven by Lasheen [8] and have thus solved this problem via selectively leaching the silica (9% in the original slag) by NaOH in a manner to obtain a TiO2 of 97% purity.
In the field of NaOH application for titanium feedstocks, it is interesting to refer to Zhang et al. [9] who have subjected titania slag to caustic hydrothermal decomposition in an autoclave using 10M NaOH Kg-1 for 4 h at 220°C and the autogenous pressure. The obtained solid product has then been stirred in water at 80°C followed by drop wise acidification / cation exchange to dissolve the impurities and to obtain a TiO2 product of > 99% purity; viz:
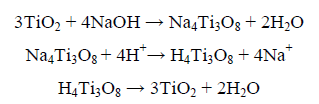
However, Manhique et al. [10] have studied ilmenite roasting with NaOH in the range of 300°C to 950°C and have indicated that different sodium iron titanates including Na2TiO3 and NaFeO2 are formed above 550°C while at 550°C and below, Na2TiO3 would be the unique binary titanate in the products. Finally, Middlemas et al. [1] have studied the production of TiO2 pigment by roasting titania slag with NaOH and after water washing and HCl leaching solvent extraction using an amine was applied to remove iron leaving a Ti - rich raffinate.
In the light of the above procedures, the present work has thus been formulated to study the alkali roasting of Rosetta titania slag followed by successive water and controlled acid leaching in order not to dissolve the titanium values. The purpose was to avoid the solvent extraction step of the resultant acid solution if it has dissolved both iron and titanium. The studied relevant factors of the suggested procedure have mainly involved the slag/NaOH ratio, the roasting temperature together with the S/L ratio in both the applied water and HCl acid leaching steps.
Experimental Procedure
Materials
A titania slag sample prepared from Rosetta ilmenite concentrate was kindly provided from the Titanium Project of the Nuclear Materials Authority (NMA). The chemical analysis of this sample is shown in Table 1. In addition, the X-ray diffraction pattern of Rosetta titania slag [8-10] has indicated that its main component is pseudobrookite as shown in Figure. 1. The stoichiometric composition of the latter is M3O5 which represents the solid solution of Fe Ti O, Ti Ti O and Ti Fe O end terms implying the occurrence of multiple valencies of Fe and Ti [11].
| Oxide | TiO2 | Fe(total) | SiO2 | MnO | Al2O3 | CaO | MgO | V2O25 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 72.00 | 12.65 | 9.00 | 1.63 | 1.80 | 0.78 | 0.60 | 0.55 | 0.32 |
Table 1. Chemical composition of rosetta titania slag.
In general, titania slag is prepared by mixing ilmenite with coal and subjected to a reduction smelting at 1500°C to 1700°C in an electric furnace where two salable products would result; namely, a low manganese high quality pig iron and a titania slag float; viz:
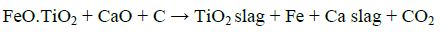
Experimental procedures
Alkaline roasting procedure: The alkaline roasting procedure that has been applied in the present work has involved 5g sample portions of the provided titania slag (− 25 mesh) that have been thoroughly mixed with different amounts of Na H in a porcelain crucible. Roasting was then achieved in a muffle furnace at the desired roasting temperature for half an hour.
Leaching procedures: Using a S/L ratio of 1/10, each alkaline roasted titania slag sample was first subjected to water leaching at its boiling temperature for 0.5 h to remove both the excess NaOH as well as the soluble impurities. The latter would mainly include the alkali silicate and aluminate as well as both the vanadium and chromium metal values. After filtration, the water-leached sample was subsequently subjected to acid leaching using 20% HCl solution under reflux at a pulp ratio (S/L) of 1/10 for 1 hr at boiling temperature. The purpose was to not leach the titanium from the treated slag sample but leaching the other impurity phases that mainly contain Fe, Mn and Ca which are water insoluble but easily dissolve in mineral acids. During all leaching procedures, the pulp was magnetically stirred at a constant rate.
Analytical procedures
Control analysis: Titanium and silica in the different stream solutions were analyzed using the respective colorimetric analytical methods. Thus, while the molybdate reagent in the presence of tartaric acid has been used for the determination of the silica content at 640 nm, the spectrophotometric determination of titanium was performed using tiron as the complexing agent and measuring the obtained absorbance at 430 nm using Unicam UV2-100 [12]. On the other hand, the total iron was titrimetrically determined against EDTA using sulfosalicylic acid as indicator [13].
Product analysis: Analysis of the main three trace elements present in the TiO2 product (Mn, Cr and V) was performed by means of the atomic absorption technique using Unicam 969 Model at 279.5 nm, 357.9 nm and 318.4 nm respectively. In the meantime, analysis of calcium and magnesium were titrimetrically analyzed using EDTA while sodium was analyzed by flame photometry.
Results and Discussion
Results of alkaline roasting of titania slag
Effect of slag/NaOH ratio: To study the effect of slag/NaOH weight ratio upon the silica and iron leaching efficiencies from the study roasted titania slag, several experiments were performed using a slag/NaOH ratio varying from 5/1 to 5/8. As mentioned above, the other roasting conditions were fixed at a roasting temperature of 850°C for 0.5 h and the roasted samples were then successively subjected to water and 20% hydrochloric acid leaching.
From the obtained results summarized in Table 2 and collectively plotted in Figure. 2, it is obvious that while silica is mostly leached by water, iron has not been leached by water whereas both iron and the remaining silica have been leached to varying degrees in HCl solution. It is also ascertained that the leaching efficiency of both silica and iron is directly proportional to the slag/NaOH ratio. Thus, at a slag/NaOH ratio of 5/6, complete leaching of iron was attained and about 95% silica leaching with only a minor amount of titanium (0.55%). By increasing the slag/NaOH ratio to 5/8, leaching of silica has actually increased to reach up to about 99%. The following possible reactions would represent the formation of the sodium titanates as well as the sodium silicate and aluminate; viz:
Figure 2: Effect of slag/NaOH ratio upon the leaching efficiency of silica in water and HCl acid and that of iron in HCl acid from the working titania slag/NaOH sinter.
| Slag/NaOH ratio, g/g | Water leaching, % | HCl leaching, % | Total Silica leaching, % | Total Iron leaching, % | ||
|---|---|---|---|---|---|---|
| Silica | Iron | Silica | Iron | |||
| 5/1 | 23.78 | 0 | 2.89 | 67. 51 | 26.67 | 67. 51 |
| 5/2 | 26.22 | 0 | 7.56 | 77.87 | 33.78 | 77.87 |
| 5/3 | 32.78 | 0 | 10.33 | 86.00 | 43.11 | 86.00 |
| 5/4 | 51.00 | 0 | 10.67 | 92.89 | 59.10 | 92.89 |
| 5/5 | 65.11 | 0 | 11.11 | 96.84 | 76.22 | 96.84 |
| 5/6 | 83.00 | 0 | 12.22 | 100.00 | 95.22 | 100.00 |
| 5/8 | 86.33 | 0 | 12.44 | 100.00 | 98.77 | 100.00 |
Table 2. Effect of slag/NaOH ratio upon the leaching efficiency of silica in water and HCl acid and that of iron in HCl acid from the working titania slag/NaOH sinter
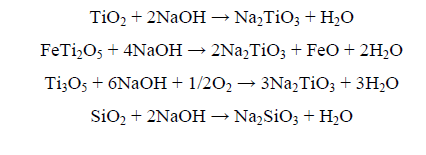
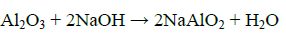
At the same time, any vanadium and chromium constituents associated with the titania slag would be converted to their water-soluble sodium metavanadate and chromate [8].
Effect of roasting temperature: In order to study the effect of the roasting temperature upon silica and iron leaching efficiencies from the working Rosetta titania slag, several experiments were carried out using roasting temperatures ranging from 500 to 900°C. The other conditions were fixed at a slag/NaOH weight ratio of 5/6 for 0.5 h and the roasted samples were then subjected to water and 20% hydrochloric acid leaching.
From the obtained results shown in Table 3 and collectively plotted in Figure. 3 it is clear that by increasing the roasting temperature from 500°C to 850°C, the silica leaching efficiency has increased from about 63% to about 95% while that of iron has increased from about 31% to 100% due to the decomposition of the slag constituents and the formation of compounds which easily respond to water and acid leaching. Increasing the roasting temperature to 900°C, the leaching of both silica and iron has slightly been decreased to 93.7 and 98.7 respectively. This decrease is most probably due to the resultant cake that has become tough and hardly leached.
Figure 3: Effect of roasting temperature upon the leaching efficiency of silica in water and HCl acid and that of iron in HCl acid from the working titania slag/NaOH ratio of 5/6.
| Roasting temperature, °C | Water leaching, % | HCl leaching, % | Total Silica leaching, % | Total Iron leaching, % | ||
|---|---|---|---|---|---|---|
| Silica | Iron | Silica | Iron | |||
| 500 | 54.00 | 0 | 8.78 | 31.41 | 62.78 | 31.41 |
| 600 | 59.67 | 0 | 9.56 | 56.74 | 69.23 | 56.74 |
| 700 | 66.33 | 0 | 10.44 | 75.33 | 76.77 | 75.33 |
| 800 | 73.56 | 0 | 11.00 | 90.62 | 84.56 | 90.62 |
| 850 | 83.00 | 0 | 12.22 | 100.00 | 95.22 | 100.00 |
| 900 | 81.80 | 0 | 11.90 | 98.70 | 93.70 | 98.70 |
Table 3. Effect of roasting temperature upon the leaching efficiency of silica in water and HCl acid and that of iron in HCl acid from the working titania slag/NaOH ratio of 5/6.
Results of S/L ratio upon water and acid leaching
In a trial to decrease the required volume of water and of 20% HCl upon silica and silica/iron leaching efficiencies from the working alkali - roasted Rosetta titania slag, two successive series of leaching experiments have been performed using different solid/liquid ratios. In other words, after water leaching of the working sinter and its filtration, it was subjected to the 20% HCl acid leaching.
Effect of S/L ratio upon water leaching of silica: The effect of solid/water (S/L) ratio upon silica leaching was investigated from 1/4 to 1/12 keeping the other parameters constant at the optimum roasting temperature of 850°C for 0.5 h and using the optimum slag/NaOH ratio of 5/6. The water leaching experiments were performed as previously mentioned i.e., at the boiling temperature and for 0.5 h.
From the obtained results plotted in Figure. 4, it is clearly evident that silica leaching is directly proportional to the decrease in the S/L ratio. Thus, decreasing the S/L ratio from 1/4 down to 1/10 has led to a progressive increase in the silica leaching efficiency from about 47 to 83% respectively. By further decreasing the S/L ratio to 1/12, only a slight increase in the silica leaching was obtained.
Figure 4: Effect of S/L ratio upon the leaching efficiency of silica in water from the working Titania slag/NaOH sinter.
On the other hand, in the water leaching experiments at boiling in 1/10 S/L ratio for 0.5 h, the NaOH consumed during the slag roasting at 850°C for 0.5 h in the slag/NaOH ratios of 5/1 to 5/8 was determined. From the obtained results summarized in Table 4. it is obvious that the sodium hydroxide consumption has actually increased from 0.74 to 2.74g/5g sample as the slag/NaOH ratio was decreased from 5/1 down to 5/6. However, further decreasing the ratio to 5/8, the NaOH consumption was increased by about 0.4 g NaOH/5g slag.
| Slag/NaOHratio, wt. ratio | 5/1 | 5/2 | 5/3 | 5/4 | 5/5 | 5/6 | 5/8 |
| Consumption,g/5g sample | 0.74 | 1.29 | 1.42 | 1.93 | 2.52 | 2.74 | 3.16 |
Table 4. Effect of slag/NaOH ratio upon the NaOH consumption.
Effect of S/L ratio upon acid leaching of iron: The effect of solid/HCl (S/L) ratio upon iron leaching was studied using S/L ratio ranging from 1/4 to 1/10. The other conditions were fixed at slag/NaOH ratio of 5/6 at roasting temperature 850°C for 0.5 h. Also, the roasted samples were then water leached using S/L ratio of 1/10 at boiling for 0.5 h, followed by acid leaching with 20% HCl at boiling for 1 h.
From the obtained results plotted in Figure. 5, it is clear that iron leaching is directly proportional to the decrease in the S/L ratio. Decreasing S/L ratio from 1/4 to 1/10 lead to complete leaching of iron. Therefore, the 1/10 ratio would be considered as the optimum S/L ratio for total iron leaching from the working sinter by 20% HCl.
Figure 5: Effect of S/L ratio upon the leaching efficiency of iron in 20% HCl from the working titania slag/NaOH sinter.
| Oxide | TiO2 | SiO2 | CaO | MgO | Na2O | MnO | Cr2O3 | V2O5 |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 99.10 | 0.40 | 0.16 | 0.10 | 0.20 | 0.002 | 0.002 | 0.001 |
Table 5. Chemical composition of the TiO2 product from rosetta titania slag by alkali roasting.
Titanium product calcination and analysis
During water and acid leaching of the roasted slag, the product sodium titanate and possible sodium iron titanates would be decomposed and hydrolyzed to TiO2. The possible reactions can be represented as follows:
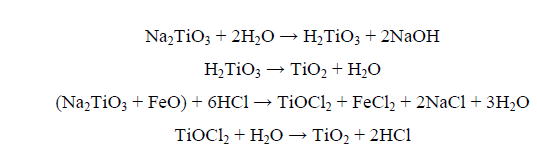
The obtained TiO2 product has actually been filtered and properly washed with distilled water before drying at 110°C. The dried product was then subjected to calcination at 850°C for 1 h in a muffle furnace. A representative portion of the TiO2 product has then been subjected to chemical analysis and the obtained results are summarized in Table 5. From the latter, it was found that the obtained TiO2 product attains a purity of 99.1% and was found to be devoid of iron. Finally, all the applied procedures upon the working Rosetta titania slag have been formulated in a generalized flowsheet as shown in Figure. 6.
Figure 6: An integrated flowsheet for the production of high grade TiO2 from Rosetta titania slag via alkali roasting.
Conclusion
The Rosetta titania slag assaying 72% TiO2 has been subjected to alkali roasting using sodium hydroxide and the relevant factors have been studied. Accordingly, it was found optimum to fix the roasting conditions as a slag/NaOH ratio of 5/6 at 850°C for 0.5 h. The obtained sinter was first treated with water at boiling temperature using a S/L ratio of 1/10 for 0.5 h to solubilize most of the sodium salts of silica and alumina together with vanadium and chromium besides the excess alkali.
In a second leaching circuit, 20% HCl solution was used under reflux at a pulp ratio (S/L) of 1/10 for 1 h to leach the remaining silica and the other impurity phases as mainly represented by Fe, Mn and Ca which are water insoluble. Analysis of the TiO2 product left behind after drying and calcination at 850°C for 1 h has indicated 99.1% purity.
Acknowledgement
The author would like to express his great thanks to Prof. Dr. N. El-Hazek, Nuclear Materials Authority (NMA) for his assistance, valuable scientific help, and revising of the manuscript.
References
- Middlemas S, Fang ZZ, Fan P. A new method for production of titanium dioxide pigment. Hydrometallurgy. 2013;131–132: 107–13.
- Roche EG, Stuart AD, Grazier PE. Production of Titania. 2004;WO2004035841-A1.
- Lakshmanan VI, Sridhar R, Harris GB, et al. Process for the recovery of titanium in mixed chloride media. 2010;US Patent 7803336.
- Verhulst D, Sabachy B, Spitler T, et al. The Altair TiO2 pigment process and its extension into the field of nanomaterials. CIM Bulletin ProQuest Science Journals 2002;95(1065);89-94.
- Foley E, MacKinnon KP. Alkaline roasting of ilmenite. J. Solid State Chem. 1970;1(3–4):566–75.
- Elger GW, Holmes RA. Purifying titanium-bearing slag by promoted sulfation. US Patent. No. 4,362,557.
- Lahiri A, Jha A. Kinetics and reaction mechanism of soda ash roasting of ilmenite ore for the extraction of titanium dioxide. Metall Mater Trans B Process Metall Mater Process Sci 2007;38(6);939–48.
- Lasheen TA. Soda ash roasting of Titania slag product from Rosetta ilmenite. Hydrometallurgy 93(3-4)124-28.
- Zhang Y, Qi T, Zhang Y. A novel preparation of titanium dioxide from titanium slag. Hydrometallurgy. 2009;96(1);52-65.
- Manhique Arao J, Focke Walter W. Titania recovery from low-grade titanoferrous minerals. Hydrometallurgy 2011;109(3);230-36.
- Dondi M, Cruciani G, Balboni E, et al. Titania slag as a ceramic pigment. Dyes Pigm. 2008;77(3):608-13.
- Marczenko Z. Separation and spectrophotometric determination of elements. Chichester Horwood; Halsted Press, New York, 1986.
- Vogel AI. A textbook of quantitative inorganic analysis. 4th ed, Longman Publications, London, 1978; pp. 742-750.

