Short communication
, Volume: 12( 3)Advantages of the Ultrasound-Assisted Compartment Reactor for Biodiesel Production
- *Correspondence:
- Imai M, Course in Bioresource Utilization Sciences, Graduate School of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa, Kanagawa Pref, Japan, Tel: +81-466-84-3978; E-mail: XLT05104@nifty.com
Received: September 12, 2017; Accepted: September 27, 2017; Published:September 30, 2017
Citation: Nakayama R, Imai M, Woodley JM. Advantages of the Ultrasound-Assisted Compartment Reactor for Biodiesel Production. Res Rev Biosci. 2017;12(3):127
Abstract
Biodiesel (fatty acid methyl esters, FAME) is an important biofuel for the future. It is produced by transesterification of triglycerides, in vegetable oils and animal fats. In the first-generation processes, alkaline-based catalysis was mostly employed. It has been established for the conversion of vegetable oils with relatively low free fatty acid content [1,2]. In second generation processes, enzymatic reaction was commonly applied, using a lipase (EC 3.1.1.3) [3-5]. Enzymatic method is anticipated as an environmental friendly processes and lower consumption of thermal energy. Several scientific reports have discussed the use of immobilized lipases. For establish lower cost in application, direct use of liquid soluble lipase is favorable for commercial processing. Several recent publications attest to the clear advantages of liquid soluble in application. Callera Trans L™ contained Thermomyces lanuginosus (Novozymes A/S, Denmark) has been used often in investigations. For an effective commercial operation, physicochemical properties of aqueous-oil interfacial area are important subject since lipases are activated at the liquid-liquid interface.
Commentary
Biodiesel (fatty acid methyl esters, FAME) is an important biofuel for the future. It is produced by transesterification of triglycerides, in vegetable oils and animal fats. In the first-generation processes, alkaline-based catalysis was mostly employed. It has been established for the conversion of vegetable oils with relatively low free fatty acid content [1,2]. In second generation processes, enzymatic reaction was commonly applied, using a lipase (EC 3.1.1.3) [3-5]. Enzymatic method is anticipated as an environmental friendly processes and lower consumption of thermal energy. Several scientific reports have discussed the use of immobilized lipases. For establish lower cost in application, direct use of liquid soluble lipase is favorable for commercial processing. Several recent publications attest to the clear advantages of liquid soluble in application. Callera Trans L™ contained Thermomyces lanuginosus (Novozymes A/S, Denmark) has been used often in investigations. For an effective commercial operation, physicochemical properties of aqueous-oil interfacial area are important subject since lipases are activated at the liquid-liquid interface.
The interfacial area and frequency of collision are depended upon the mixing condition. It has been conventionally carried out using a stirrer. Ultrasound irradiation was proposed as attractive alternate technique to enhance reactivity yield. Several reports demonstrated effective improvements in lipase-based reactions via the use of ultrasound. Cavitation caused by ultrasound increased intensity of turbulence and micro-scaled circulation currents. They are effective tool for elimination of the mass transfer resistances [6-9].
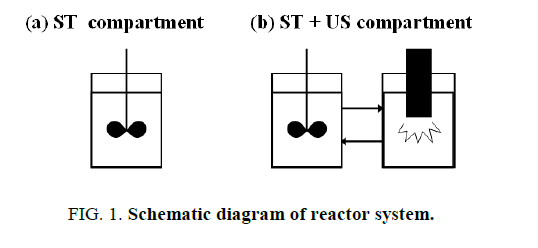
We have reported some attractive results using a two-compartment reactor [10]. The reactor was composed of a mechanically stirred compartment (ST) and an ultrasound irradiation compartment (US). The reaction solution was recirculated between the ST and the US. The reactor demonstrates the enhancement effect of ultrasound on the enzymatic reaction. In this article, the effect of ultrasound irradiation and stirring (Figure. 1) were shown by using substrates rapeseed oil and methanol, soluble lipase (Callera L™).
Rapeseed oil, from Emmelev A/S (Otterup, Denmark), methanol (99.8%, technical grade), and soluble lipase (CalleraL TM) which was kindly donated by Novozymes A/S (Bagsværd, Denmark) were employed.
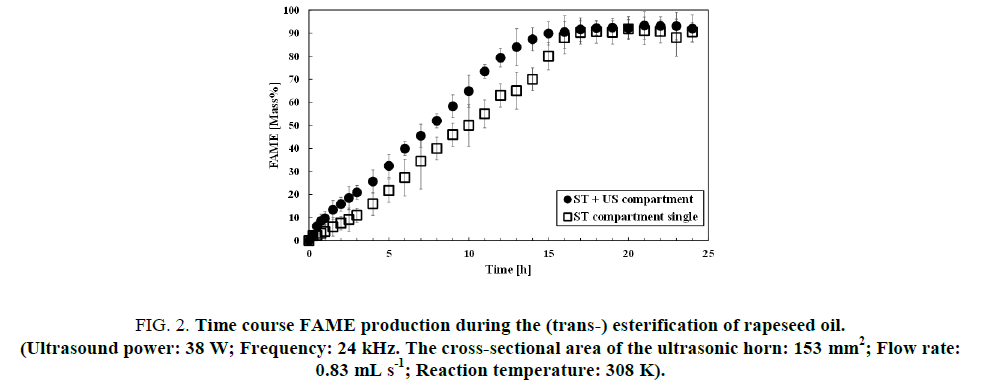
Figure. 2 depicted the time course of FAME production. In this study, examinations were carried out triplicate repeating and the mean values were presented. The initial reaction rate in the ST+US compartment was 1.3 folds higher than that of the ST single system. Numerical evidence of ultrasound irradiation in the separate vessel (US) was our original data for forthcoming development of industrial FAME production.
Figure 2: Time course FAME production during the (trans-) esterification of rapeseed oil.
(Ultrasound power: 38 W; Frequency: 24 kHz. The cross-sectional area of the ultrasonic horn: 153 mm2; Flow rate: 0.83 mL s-1; Reaction temperature: 308 K).
Reaction rate was improved by the aid of ultrasonication in US compartment. Micro-scale turbulence caused by cavitation reduced mass transfer resistance.
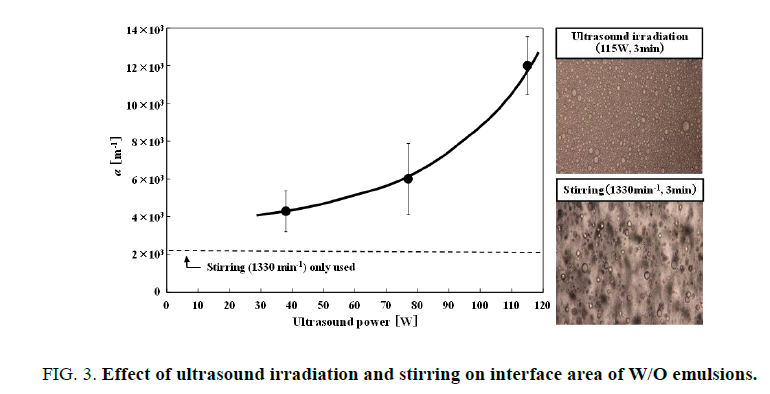
Ultrasound irradiation strongly influenced mean diameter of W/O emulsion droplet. Specific interfacial area (α [m-1]) in the W/O emulsion with ultrasound power was presented in Figure. 3. The Sauter mean diameter of W/O emulsion droplets d32 was determined by measurement of one hundred droplets. The specific interface area of W/O emulsions calculated by eqn. (1).
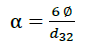 (1)
(1)
The volumetric fraction of water phase φ was constant at 0.02. Amphiphilic reagent Span 80 (0.01 wt %) was limitedly used in measuring droplet size. In the case of ultrasound irradiation, the interfacial area of W/O emulsion was apparently increased with respect to the stirring only used.
Promising effect of ultrasound irradiation on improvement of FAME production rate was brought by the elimination of mass transfer residence by microscale turbulence and the production of large interfacial area in reaction liquid phase.
References
- Freedman B, Pryde EH, Mounts TL. Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc. 1984;61:1638-43.
- Ma F, Hanna MA. Improvement of biodiesel product yield during simple consecutive-competitive reactions. Bioresource Technol. 1999;70:1-15.
- Fjerbaek L, Christensen KV, Norddahl B. A review of the current state of biodiesel production using enzymatic transesterification. Biotech Bioeng. 2009;102:1298-15.
- Wang M, Nie K, Yun F, et al. Biodiesel preparation by lipase-catalyzed transesterification of Jatropha oil. Renew Energy. 2015;83:1020-25.
- Nordblad M, Silva VTL, Nielsen PM, et al. Identification of critical parameters in liquid enzyme-catalyzed biodiesel production. Biotech Bioeng. 2014;111:2446-53.
- Teixeira CB, Junior JVM, Macedo GA. Agility in energy-Shaping the consumer-centric energy world. Renewable and Sustainable Energy Reviews. 2014;33:333-43.
- Bradley M, Ashokkumar M, Grieser F. Sonochemical Production of Fluorescent and Phosphorescent Latex Particles. J Am Chem Soc. 2003;125:525-29.
- Adewale P, Dumon MJ, Ngadi M. Castor oil transesterification catalysed by liquid enzymes: Feasibility of reuse under various reaction conditions. Ultrason Sonochem. 2015;27:1-9.
- Subhedar PB, Botelho C, Riberio A, et al. Hidroesterificação de Gordura Abdominal de Frango Catalisada pela Lipase NS-40116. Ultrason Sonochem. 2015;27:530-535.
- Nakayama R, Imai M, Woodley JM. Ultrasound-assisted production of biodiesel FAME from rapeseed oil in a novel two-compartment reactor. J Chem Technol Biotechnol. 2017;92:657-665.