Original Article
, Volume: 12( 3)A Solvent Free Green Protocol for Synthesis of 5-Arylidine Barbituric Acid Derivatives
- *Correspondence:
- Uttam BM, Department of Chemistry, Sadguru Gadage Maharaj College, Karad, India, E-mail: uttambmore@rediffmail.com
Received: July 07, 2016; Accepted: July 20, 2016; Published: July 26, 2016
Citation: Uttam BM. A Solvent Free Green Protocol for Synthesis of 5-Arylidine Barbituric Acid Derivatives. Org Chem Ind J. 2016;12(3):102.
Abstract
A green approach to the condensation reaction of aromatic aldehydes and barbituric acid has been described using sodium acetate as a catalyst with grinding at room temperature without using solvent. Short reaction time, higher yields and clean reaction makes this protocol an attractive alternative to the existing methods.
Keywords
Grinding; Barbituric acid; 5-arylidine barbituric acid
Introduction
Barbituric acid is a strong acid in aqueous medium with an active methylene group and can be involved in Knoevenagel type condensation reaction. Barbituric acid is a cyclic amide used as the parent compound to produce barbiturates that act as central nervous system depressants. Barbituric acid itself does not give sedative and hypnotic effects but the substituted derivatives with alkyl or aryl group at position 5 provide effects. The derivatives of barbituric acid have special place in pharmaceutical chemistry. They have broad biological spectrum ranging from classical applications in medical treatments as anticonvulsant, sedative, antiplasmodic, hypnotic and local anesthetic drugs [1,2]. They have been also found useful in anti-osteoporosis, anti-tumor and anti-cancer treatments [3,4].
Different synthetic routes have been reported for the synthesis of 5-arylidine barbituric acid derivatives by using NH2SO3H [5], infra-red promoted [6], microwave irradiation [7], ionic liquid mediated condensation [8], and uses variety of catalysts such as ZnCl2 [9], CdI2 [10], Ni-SiO2[11], KF–Al2O3 [12], natural phosphate [(NP)/KF or NP/NaNO3] [13] and synthetic phosphate (Na2CaP2O7) [14], K2NiP2O7 [15], dry condensation with acidic clay catalysts [16], Ni nanoparticles [17], microwave irradiation [18] etc. However, these methods are suffering by limitation of longer reaction time, effluent pollution, bis-addition and self-condensation, lower yields etc.
Solvent free organic reaction have drawn great interest, particularly from the viewpoint of green chemistry as organic solvent are toxic and flammable. Solid state reactions are simple to handle, reduce pollution and comparatively cheaper to operate.
Organic synthesis employs large amounts of hazardous and toxic solvents. The choice of pursuing aqueous reactions is becoming more and more important due to its environmental impact and cost of chemicals. Organic reactions under aqueous conditions have increasingly attracted chemist’s interests particularly from the view point of green chemistry. Organic solvents are conventionally used in organic synthesis and in industrial processes on a large scale. These solvents are often problematic owing to their toxicity and flammability. There is now a realization that more benign chemical synthesis is required, as an integral part of developing sustainable technologies. Eliminating the use of organic solvents can reduce the generation of waste, which is a requirement of one of the principles of green chemistry.
The grinding method is used more and more frequently in organic synthesis. In comparison with traditional methods, this method is more convenient and easily controlled. A number of organic reactions can be carried out in higher yields, shorter times or milder conditions by the grinding method. The synthesis of fused isoxazole derivatives has been achieved by grinding method [19]. It can even set off some reactions that cannot be carried out under traditional conditions. All of these results prompted us to study the possibility of Knoevenagel condensation of aromatic aldehydes with barbituric acid catalyzed by sodium acetate under grinding without solvent.
Experimental
All the reagents and solvents were used as purchased without further purification. Melting points were determined on a Fisher-Johns melting point apparatus and are uncorrected. IR spectra were obtained on a Perkin-Elmer BX serried FTIR spectrometer using KBr pellet. NMR spectra were recorded on 300 MHz spectrometer.
General Procedure for the Synthesis of 5-Arylidene Barbituric Acids
A mixture of aromatic aldehyde (10 mmol), barbituric acid (10 mmol) and sodium acetate (10 mmol) was grinded at room temperature till the completion of reaction. The reaction was monitored by TLC (hexane and ethyl acetate). After completion of reaction solid product was washed with distilled water, filtered and recrystallized using suitable solvent ( Scheme 1 and Table 1).
| Entry | Aldehyde | Product | Time (min) | Yield(%) | m. p. (ºC) | |
|---|---|---|---|---|---|---|
| Found | Reported [20,21] | |||||
| 1 | 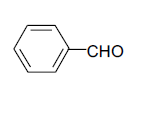 |
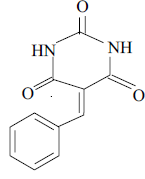 3a 3a |
07 | 86 | 265-266 | 263-265 |
| 2 | 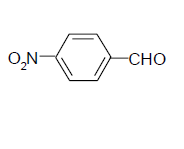 |
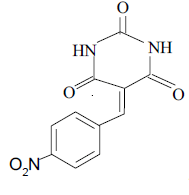 3b 3b |
15 | 81 | 268-270 | 272-274 |
| 3 | 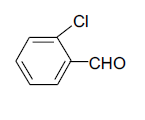 |
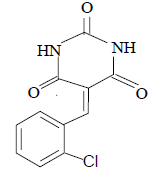 3c 3c |
07 | 90 | 248-250 | 252-254 |
| 4 | 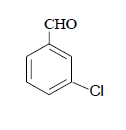 |
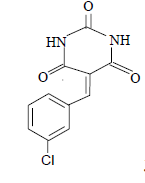 3d 3d |
15 | 85 | 276-278 | 274-278 |
| 5 | 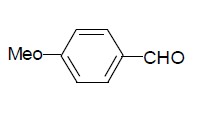 |
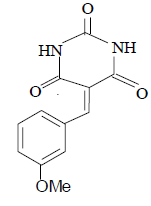 3e 3e |
10 | 87 | 295-297 | 296-298 |
| 6 | 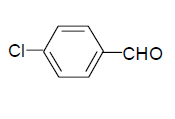 |
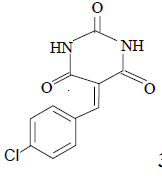 3f 3f |
10 | 91 | 296-798 | 301-302 |
| 7 | 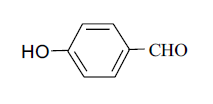 |
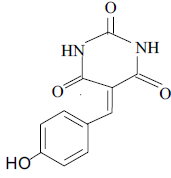 3g 3g |
15 | 83 | >300 | >320 |
| 8 | 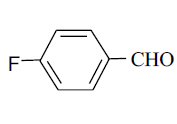 |
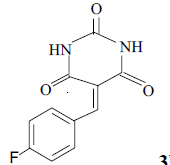 3h 3h |
20 | 77 | >300 | 309-310 |
| 9 | 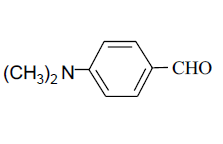 |
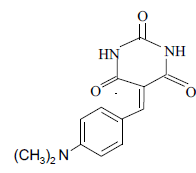 3i 3i |
15 | 82 | 274-276 | 277-279 |
| 10 | 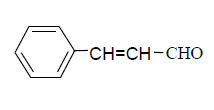 |
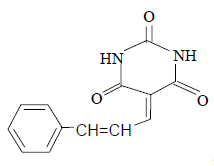 3j 3j |
10 | 84 | 268-270 | 270 |
| 11 | 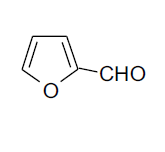 |
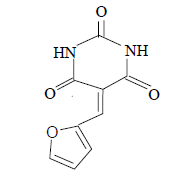 3k 3k |
15 | 76 | 260-262 | 264 |
| 12 | 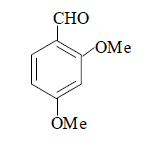 |
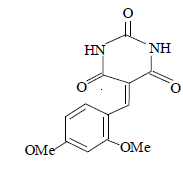 3l 3l |
10 | 84 | 285-287 | 288-290 |
| 13 | 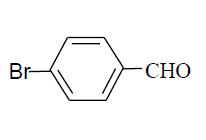 |
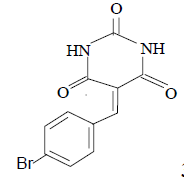 3m 3m |
20 | 77 | 288-290 | 292-293 |
Table 1: Condensation of barbituric acid with aromatic aldehydes catalyzed by sodium acetate using grinding method.
Spectral data of some compounds
3c: IR (KBr) υ cm-1: 3450, 3126, 2978, 1728, 1562, 1450, 1073; 1H NMR: (300 MHz, DMSO-d6) δ: 7.30 (t, 1H Ar-H), 7.41 (t, 1H, Ar-H), 7.48 (d, 1H, Ar-H), 7.68 (d, 1H, Ar- H), 8.21 (s, 1H, HC=C), 11.15 (s, 1H, NH), 11.41(s, 1H, NH).
3d: IR (KBr) υ cm-1: 3255, 3210, 2970, 1730, 1570, 1440, 1078; 1H NMR: (300 MHz, DMSO-d6) δ: 7.51-7.01 (m, 2H Ar-H), 7.78 (d, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 8.26 (s, 1H, CH=), 11.16 (s, 1H, NH), 11.22 (s, 1H, NH).
3e: IR (KBr) υ cm-1: 3210, 3052, 2965, 1728, 1692, 1650, 1552; 1H NMR: (300 MHz, DMSO-d6) δ: 3.80 (s, 3H, MeO), 7.01 (d, 2H Ar-H), 7.86 (t, 1H, Ar-H), 8.16 (s, 1H, CH=), 8.30 (s, 1H, Ar- H), 11.10 (s, 1H, NH), 11.16 (s, 1H, NH).
3f: IR (KBr) υ cm-1: 3414, 3210, 2965, 1745, 1700, 1564; 1H NMR: (300 MHz, DMSO-d6): δ: 7.45 (d, 2H, Ar-H), 8.18 (2d, 2H, Ar-H), 8.16 (s, 1H, HC=), 11.18 (s, H, NH), 11.44 (S, 1H, NH).
3g: IR (KBr) υ cm-1: 3412, 3214, 2968, 1732, 1706, 1572; 1H NMR: (300 MHz, DMSO-d6): δ: 6.80 (d, 2H, Ar-H), 8.28 (2d, 2H, Ar-H), 8.20 (s, 1H, HC=), 10.63 (S, 1H, OH), 1.10 (s, H, NH), 11.19 (S, 1H, NH).
3j: IR (KBr) υ cm-1: 3220, 3068, 2966, 2866, 1601, 1726, 1510, 1418, 1370, 1292; 1H NMR: (300 MHz, DMSO-d6) δ: 7.40-7.62 (m, 5H Ar-H), 7.61 (d, 1H, HC=), 7.12 (d, 1H, HC=), 11.12 (s, 1H, NH), 11.23 (s, 1H, NH).
Results and Discussion
We have described mild, easy and green protocol for the synthesis of 5-arylidine barbituric acid derivatives using sodium acetate at room temperature under grinding condition. Short reaction times, appropriate yields and clean reactions make this procedure an attractive alternative to the existing methods. Furthermore, this method is of interest in the perspective of environmentally greener and safer method.
Acknowledgement
Author is thankful to Dr. Mohan Rajmane, Principal, Sadguru Gadage Maharaj College, Karad Dist., Satara (MS), India for providing laboratory facility for research work.
References
- Tanka K, Cheng X, Yoneda F. Oxidation of thiol with 5-arylidene-1, 3-dimethylbarbituric acid: application to synthesis of unsymmetrical disulfide. Tetrahedron. 1988;44:3241-9.
- Sokmen BB, Ugras S, Sarikaya HY, et al. Antibacterial, Antiurease, and Antioxidant Activities of Some Arylidene Barbiturates. App Biochem Biotech. 2013;171(8):2030-9.
- Jones G. The Knoevenagel condensation reaction in organic reactions. Vol. 15. New York: John Wiley; 1967. p. 204-599.
- Khajuria RK, Jain SM, Dhar KL. Studies On Cycloaddition Reactions of 2,4-Dihydroxy-3-Undecyl-1,4-Benzoquinone-(Embelin) and 2,3-Dimethylbuta-1,3-Diene. Indian J Chem. 1996;35:860-1.
- Li JT, Dai HG, Liu D, et al. Efficient Method for Synthesis of the Derivatives of 5‐Arylidene Barbituric Acid Catalyzed by Aminosulfonic Acid with Grinding. Synth Commun.2006;36:789-94.
- Alarreca G, Sanabria R, Miranda R, et al. Preparation of benzylidene barbituric acids promoted by infrared irradiation in absence of solvent. Synth Commun. 2000;30:1295-1301.
- Dewan S, Singh R. One pot synthesis of barbiturates on reaction of barbituric acid with aldehydes under microwave irradiation using a variety of catalysts. Synth Commun. 2003;33:3081-4.
- Wang C, Ma J, Zhou X, et al. 1‐n‐Butyl‐3‐Methylimmidazolium Tetrafluoroborate–Promoted Green Synthesis of 5‐Arylidene Barbituric Acids and Thiobarbituric Acid Derivatives. Synth Commun.2005;35:2759-64.
- Rao PS, Venkataratnam RV. Zinc chloride as a new catalyst for Knoevenagel condensation.Tetrahedron Lett.1991;32:5821-2.
- Prajapati D, Sandhu JS. Cadmium iodide as a new catalyst for knoevenagel condensations. JChemSoc Perkin Trans.1993;1(6):739-40.
- Pullabhotla Rajasekhar VSR, Rahman A, Jonnalagadda SB, Selective catalytic Knoevenagel condensation by Ni–SiO2 supported heterogeneous catalysts: an environmentally benign approach. CatalCommun.2009;10:365-9.
- Dai G, Shi D, Zhou L, et al. Knoevenagel Condensation Catalysed by Potassium Fluoride/Alumina. Chinese JApplChem.1995;12:104-8.
- Sebti S, Smahi A, Solhy A. Natural phosphate doped with potassium fluoride and modified with sodium nitrate: efficient catalysts for the Knoevenagel condensation. Tetrahedron Lett.2002;43:1813-6.
- Bennazha J, Zahouily M, Sebti S, et al. A Na2CaP2O7, a new catalyst for Knoevenagel reaction. CatalCommun.2001;2:101-4.
- Maadi EA, Matthiesen CL, Ershadi P, et al. Knoevenagel condensation catalyzed by K2NiP2O7. Synthesis of (E)-methyl-α-cyanocinnamates in high yields. JChemCryst. 2003;33(10):757-63.
- Deb ML, Bhuyan PJ. Uncatalysed Knoevenagel condensation in aqueous medium at room temperature. Tetrahedron Lett.2005;46:6453-6.
- Khurana J, Vij K. Nickel Nanoparticles Catalyzed Knoevenagel Condensation of Aromatic Aldehydes with Barbituric Acids and 2-Thiobarbituric Acids. CatalLett.2010;138(1):104-10.
- Sattar MA, Khatun K, Islam R, et al. Synthesis of barbituric acid derivatives using microwave irradiation method and in vitro evaluation of antimicrobial and cytotoxic activity. J App Pharm Sci. 2015;5(11):38-42.
- Badrey MG, Gomha SM. Synthesis and Antibacterial Activity of Fused Isoxazole Derivatives Using Grinding Method. IntJPharmPharm Sci. 2014;6(7):236-9.
- Jin TS, Zhao RQ, Li TS. An Efficient and Convenient Procedure for Preparation of 5-Arylidene Barbituric Acid Catalyzed by ZrO2/SO42- Solid Superacid with Grinding. Asian J of Chem. 2007;19(5):3815-20.
- Dighore NR, Anandgaonkar PL, Gaikwad ST, et al. Solvent free green Synthesis of 5-arylidine Barbituric acid Derivatives Catalyzed by Copper oxide Nanoparticles. ResJ ChemSci. 2014;4(7):93-8.


