Research
, Volume: 18( 1)A Short Review on Schiff Bases and Applications
- *Correspondence:
- A R Rahimova
Department of General and Inorganic Chemistry,
Baku State University,
Baku,
Azerbaijan,
Tel: 9876543221;
E-mail: rahimova_aysel@mail.ru
Received: July 22, 2022, Manuscript No. TSIC-23-15938; Editor assigned: July 27, 2022, PreQC No. TSIC-23-15938 (PQ); Reviewed: August 11, 2022, QC No. TSIC-23-15938; Revised: February 15, 2023, Manuscript No. TSIC-23-15938 (R); Published: March 15, 2023, DOI: 10.4172/tsic.2023.18(1).001
Abstract
Shiff bases are condentation of primary amines with carbonyl compounds and they were first reported by Schiff in 1864. The common structural feature of these compounds is the azomethine group with a general formula RHC=N-R1, where R and R1 are alkyl, aryl, cyclo alkyl or heterocyclic groups which may be variously substituted. These compounds are also knows as anils, imines or azomethines. Several studies showed that the prescence of a lone pair of electrons in an sp2 hybridized orbital of nitrogen atom of the azomethine is group of considerable chemical and biological importance. Because of the relative easiness of preparation, synthetic flexibility, and the special property of C=N group.
Keywords
Schiff bases; Metal complexes; Antimicrobial activity
Introduction
Schiff bases are generally excellent chelating agents, especially when a functional group like OH or SH is present close to the azomethine group so as to form a five or six membered ring with the metal ion verstality of Schiff base ligands and biological, analytical and industrial applications of their complexes make further investigations in this area highly desirable. Nowadays, the research field dealing with Schiff base coordination chemistry has expanded enormously. The importance of Schiff base complexes for bioinorganic chemistry, catalysis and material science, separation and encapsulation processes, and formation of compounds with unusual properties and structures has been recognized and reviewed. Schiff bases resulted from aromatic aldehydes ortho-substituted with a hydroxyl group have initially arouse the researchers interest because of their ability to act as bidentante ligands for transitional metal ions [1]. Later, in studies concerning quantitative structure antitumor activity relationship of a series of Schiff bases derived from variously substituted aromatic amines and aldehydes, it has been shown that azomethines from salicylaldehydes gave the best correlation. Schiff bases of salicylaldehydes have also been reported as plant growth regulators and antimicrobian or antimycotic activity. Schiff bases also show some analytical applications. Schiff Bases are characterized by the N=CH-(imine) which imports in elucidating the mechanism of transamination and rasemination reaction in biological system. Schiff bases are active against a wide range of organisms for example: Candida albicans, Esherichia coli, Stapylococas aureus, Baccillus polmxa, Trychophyton gypseum, Mycobacteria, Erysiphe gramminis and Plasmopora viticola. A large number of different [2].
Schiff base ligands have been used as cation carriers in potentiometric sensors as they have shown excellent selectivity, sensitivity, and stability for specific metal ions such as Ag(2), Al(3), Co(2), Cu(2), Gd(3), Hg(2), Ni(2), Pb(2), Y(3), and Zn. Schiff bases have been studied for their important properties in catalysis. They show catalytic activity in hydrogenation of olefins. They find applications in biomimetic catalytic reactions. An interesting application of Schiff bases is their use as an effective corrosion inhibitor, which is based on their ability to spontaneously form a mono layer on the surface to be protected. Many commercial inhibitors include aldehydes or amines, but presumably due to the C=N bond the Schiff bases function more efficiently in many cases. The principial interaction between the inhibitor and the metal surface is chemisorptions. The inhibitor molecule showed have centers capable of forming bonds with the metal surface by election transfer. In such cases the metal acts as an electrophile and the inhibitor acts as a lewis base. Nucleophilic centers, such as oxygen and nitrogen atoms, of the protective compound have free electron pairs which are readily available for sharing. Together with the atoms of the benzene rings they create multiple absorption sites for the inhibitor thus enabling stable monolayer formation. Imines also have biological importance. An imine linkage between the chemistry of vision [3]. Vitamins are also called coenzymes, meaning that they are to the functioning of many enzymes, which are large proteins that catalyze chemical changes in cell. An example of a biologically important aldehyde is pyridoxal phosphate, which is the active form of the vitamin B6. Vitamin B6 serves as a coenzyme, by forming an imine with an amino acid grouping an enzyme. The coenzyme, bound to the enzyme, is involved in transamination reaction, the transfer of the amino group from one amino acid to another, which is important in the metabolism and the biosynthesis of amino acids. In the last step, enzyme-catalyzed hydrolysis cleaves the imine to pyridoxol and the modified amino acid. Schiff bases have been reported in their biological properties, such as antibacterial, antifungal activities. Their metal complexes have been widely studied because they have anticancer and herbicidial applications. They serve as models for biologically important species.
Literature Review
Report of synthesis and apllications
Three new series of biologically active amino substituted Schiff bases with general formula, R1N=CHR2. Here R1=2-aminobenzsthiazole, 4-amino-salicyclic acid and aminophenol. R2=4-chloro-benzaldehyde, 2-chloro-benzaldehyde, salicyclaldehide, vanilli and benzaldehyde were synthesized by the reaction of three different amino substituted compounds were characterized by different physic-chemical techniques like, melting point, elemental analysis, multinuclear NMR (1H, 13C). The free ligands and their metal complexes have been screened for their in vitro biological activities against bacteria, fungi and yeast. The metal complexes show more potent activities compared with Schiff base ligands. These compounds exhibited significant activity against all the tested microorganisms [4]. The scientist of Damascus university have been synthesized 1,4-Bis(3-aminopropyl)- piperazine was condensed with various aromatic aldehyde in ethanol in the prescence of acetic acid as catalyst to yield the Schiff base. These Schiff bases on treatment with phthalic anhydride gave substituted oxazepine. In this work the inhibiting action of Schiff bases and their derivative on the corrosion steel in 1M H2SO4 solution has been investigated. The electro chemical techniques such as polarization measurements were used in this study. Differents in behavior of inhibitors were explained based on structural properties of investigated inhibitors.
It is evident from literature that isatin derivatives are known to be associated with broad spectrum of biologically activity. In view of these facts and a continuation of their work in the laboratory Indian scientists prompted as to synthesize some new 3-[(5- benzylidene-2-phenyl)-3,5-dihydro-4H-imidazol-4-one-3-(4-benzoylhydrazono)]-indole-2-ones. All the synthesized compounds were screened for their in vitro antibacterial and antifungal activity. As many as new fourteen compounds were synthesized by adopting similar above procedure and then characterized by their physical, analytical and spectral data. The details of some of the representative compounds are given in the experimental section. Their physical and elemental analysis data are presented. All the synthesized compounds were tested for in vitro antimicrobial activity by the disk diffusion technique. The tested compounds exhibited mild to moderate antibacterial activity against all strains of bacteria. The compounds tested against S. aureus, showed highest activity. It has also been observed that compounds showed activity against B. subtilis. All the synthesized compounds were tested for in vitro antimicrobial activity by the disk diffusion technique. The antimicrobial study revealed that substitution in the 5th position of isatin with chlorine, bromine or fluorine produce more active compounds in a series.
Many compounds carrying 3(2H)-pyridazinone and 1(2H)-phthalazinone rings are known to have different biological activities such as antiplatelet, antihypertensive, analgesic, and anti-inflammatory actions. However, some compounds bearing 3(2H)- pyridazinonone or 1(2H)-phthalazinone ring have been reported to have antimicrobial activity. In addition, some benzene sulfonohydrazide derivatives have been reported to have antibacterial activity [5]. On the basis of these findings the scientist of Gazi university have been synthesized some 3(2H)-pyridazinon and 1(2H)-phthalazinon derivatives. The synthesized compounds were evaluated for their antibacterial activity against various gram-positive and gram-negative strains of bacteria and their clinical isolates and for their antibacterial activity against M. tuberculosis H37Rv. The results showed that that synthesized compounds were generally compounds possessing higher activities (Figure 1).
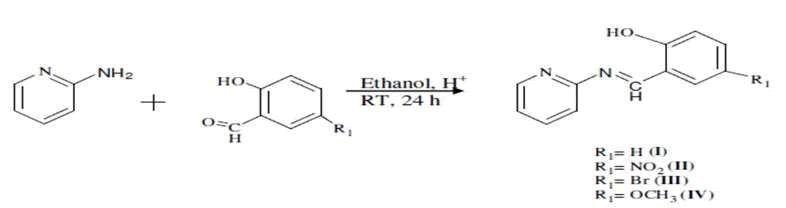
This has led to concentrate deep research on this class of compounds and their metal complexes. Similarly, the presence of hetero-atoms in the Schiff bases enhances activity. On the basis of Indian scientists work to understand the role of fine electronic variations on molecular activity and the effect of substituent location in salicylidene-2-aminopyridine Schiff bases on the absorption spectra in inorganic solvents of changeable polarities and their antibacterial activity against some common pathogens against some common pathogens namely Staphylococous aureus, Entercoccus feacalis, Pseudomonas aeruginosa and Esherichia coli. On this bases scientists have been synthesized (1) N-(2-hydroxylbenzylidene) pyridine-2-amine, (2) N-(5-nitro-2- hydroxylben-zyliddene) pyridine-2-amine, (3) N-(5-bromo 2-hydroxylbenzylidene) pyridine-2amine, (4) N-(5-metoxy-2- hydroxylbenzylidene pyridin-2-amine are prepared from 2-aminopyridine and substituted benzyaldehydes. The synthesized compounds are characterized, (4) N-(5-metoxy-2-hydroxylbenzylidene pyridin-2-amine are prepared from 2-aminopyridine and substituted benzyaldehydes [6]. The growth prevention capability was affected by the solvent and substitute group on the salicyldene part. The compounds have the ability to preventing metabolic growth of S. aureus and E. coli to different extent. The antimicrobial activity of the compounds depends on the nature of substituted present in the nature of present on the aldehyde. The importance of this lies within the potential use of the compounds as narraw spectrum antibiotics in treatment of some common deseases (Figure 2).
The pharmacological importance of heterocycles derived from 1,2,4-triazole paved the way towards active research in a triazole chemistry and occupy a prime place in medicinal and pesticide chemistry due to their capability to exhibit a wide range of activities, such as antimicrobial, antidepressant, anticonvulsant, anticancer, antipyretic, selective enzyme inhibitory activities etc. Amongst a large array of medicinally important pyrazole derivative, 4-functionalized pyrazoles occupy a unique position and their evaluation as antimicrobial agents has attracted much attention in the past [7]. Pyrazoles with various functional groups at position-4 such as cyano or oxime, aldehyde or carboxylate have been known to show good antimicrobial properties. In view of these and in continuation of our research on biologically active molecules we hereby report the synthesis of some new Schiff bases bearing triazole and pyrazole mouties and their antibacterial studies. In the present study a series of new Schiff bases were synthesized. All the synthesized compounds were characterized by IR, HNMR, mass spectral and elemental analyses. Newly synthesized compounds were screened for their antibacterial activity. The results revealed that, compounds have exhibited significant biological activity against the tested microorganisms. Wide varieties of geometries and reactivity of semicarbozone metal complexes have been reported to possess several biocidial activity of semicarbozone have been subject of investigation in recent years [8]. The biological activity is related to their interaction with several metal ions Schiff bases ligand and their metal complexes play an important application in the area of analytical chemistry, polymer sciences, food and dyes industry, agriculture biological sciences as antimicrobial agents, medical sciences as anticancer and metal corrosion inhibition agents. In view of the growing interest in the biocidial importance of Schiff and their metal complexes and in continuation of our earlier recent work in this field we now report, the synthesis, characterization and antibacterial activity of Cu(2), Co(2), Ni(2) complexes with bidentante Schiff base 1-propyl-2-6-diphenylpiperidone semicarbazone. On the bases of this work a series of metal complexes derivatives of 1-Propyl-2-6-Diphenyl Piperidone Semicarbazone (PDRS) with metal ions Cu(2), Co(2), Ni(2) have been synthesized. The ligand and metal complexes obtained are characterized quantitatively by using, molar mass, elemental analysis, infrared spectra electronic spectra, magnetic susceptibility and conductivity measurements. On the bases of above physiochemical analysis, it has been observed that the ligand PDRS coordinate to the metal ion in a bidentate manner through azomethine nitrogen and oxygen atom of semicarbazone moiety [9]. The remaining coordination centers are satisfied by anions such as X=Cl, Br, I. Electronic spectral measurement proposed the general composition of the complexes [M (PDRS) 2X2] where M=Cu (2), X=Cl and Br, I. The complexes of Co(2), Ni(2) were proposed octahedral geometries whereas distorted octahedral geometry reported for Cu(2) complexes. The preliminary in vitro antibacterial screening activity revealed that complexes showed better inhibition against tested bacterial strains and higher compared to parent ligand (Figure 3).
Discussion
Schiff bases are the important class of compounds owing to their wide range of biological activities and industrial applications. These compounds are now used to formulate anticancer1, anti HIV2, antitubercular3, antiviral4, antimalarial5 drugs. Many potent antibacterial and antifungal agents have also been prepared. Search of novel antibacterial medicines are very much needed in present time especially for tropical countries like Bangladesh. A large number of antibacterial agents are available to manage pathogenic microorganisms in nature [10]. These treatments however could not completely destroy such organisms, probably due to the widespread irrational, unscientific and apathetic use of such agents. The survived microorganisms have matched the ingenuity in developing their own defenses. As a result such drugs gradually lose their effectiveness in action. Repetition and overdose of such drugs often cause severe environmental pollution. In order to get rid of this situation, it has become a common practice to find out safer, more effective and inexpensive new chemical compounds as antibacterial agents. In this context a series of researches with various compounds have been carried out by different workers [11].
The synthesis of unsymmetrical Schiff base has attracted more interest. Although unsymmetrical ligands can clearly offer many advantages over their symmetrical counter parts in the elucidation of the composition and geometry metal ion binding sites in metalloproteins, and in the development leading to the duplication of enzymatic efficiency and selectivity of natural systems with synthetic materials, the difficulty of preparation of such ligands has hampered progress because simple condensation methodology with three components is no longer applied. In this paper we report the synthesies, structural characterization and biological activities of Cu(2), Zn(2), Ni(2), and Mn(2) complexes of the new Schiff base ligand synthesized from 2-hydroxy-1- naphtaldehyde and 5-amino-1-naphtol. The new complexes were investigated spectropically and biological activity [12].
The Schiff base and their metal complexes have more importance recently because of their applications as biological, biochemical, analytical, antimicrobial, anticancer, antibacterial, antifungal and antitumor activity. Compounds containing the sulphonamide group have long been used as drugs for diseases sulpha drugs with aldehydes, ketons or their derivatives are biologically very active, besides having good complexing ability, their activity has also been shown to increase on complexation with metal ions. The new coordination complexes of Co(2), Ni(2) and Cu(2) have been synthesized from Schiff base derived from sulfapyridine and 2-hydroxynapthaldehyde. The nature of bonding and the structural features of the Schiff base and its complexes have been deduced from elemental analysis, molar conductance, magnetic susceptibility measurements, cyclic voltammetry. These Schiff base and its metal complexes exhibited enhances antimicrobial activity compare to uncomplexed Schiff base [13].
The chemistry of 1, 2, 4-triazole and its fused heterocyclic derivatives has received considerable attention owing to their synthetic and effective biological importance. 1,2,4-triazole moieties have been incorporated into variety of therapeutically interesting drug candidates including antiviral (ribavarin), antimigraine (rizatriptan), antifungal (flucanazole) antianxiety compounds (alprazolam). Moreover sulfur containing heterocyclic compounds represent an important group of compounds that are promising on practical application. The pharmacological importance of heterocycles derived from 1,2,4-triazole paved the way too wards active research in a triazole chemistry. Pyrazoles represent a key in hetoocyclic chemistry and occupy a prime place in medicinal and pesticide chemistry due to their capability to exhibit a wide range of bioactivities, such as antimicrobial antinflammatory antidepressant anticonvulstant antipyretic [14]. Amongst a large array of medicinally important pyrazole derivatives, 4- functionalized pyrazoles occupy a unique position and their evaluation as antimicrobial agents has attracted much attention in the past. Pyrazoles with various functional groups at position-4, such as cyano or oxime aldehyde or carboxylate have been known to show good antimicrobial propertiers. In view of these and in continuation of our research on biologically active molecules we hereby repor the synthesis of some new Schiff bases bearing triazole and pyrazole moieties and their antibacterial studies.
Some Schiff bases were tested for fungicidial activity, which is related to their chemical structure, there metal complexes are important in biochemical process. For example, the transamination reactions are catalyzed by metals ions through the formation of intermediate Schiff bases containing vitamin B6. In the area of bioinorganic chemistry interest in Schiff base complexes has centered on the role of such complexes in providing synthetic models for the metal containing sites in metalloproteins and enzymes. Schiff base ligands are potential anticancer drugs and the anticancer activity of these metal complexes are enhanced in comparison to their free ligands. 3, 4-dihydroxybenzaldehyde, (Protocatechuaaldehyde, PCA) derivatives were evaluated and showed inhibition for bacteria growth, antioxidant, antitumor, anticorrosion and reagent in simple and highly sensitive analyses of Cr.
The thiazole ring is very important in nature. It occurs for example, in thiamine, a coenzyme is required for the oxidative decarboxylation of α-keto acids. A tetra hydrothiazole also appears in the skeleton of penicillin, which is one of the first and still most important broad-spectrum antibiotics. Thiazole, thiazolamines are valuable medicines. It is obvious that compounds with the thiazole ring have a potential biological activity. Similarly 2-aminothiazoles are known as biologically active compounds with a broad range of activity and they are also used as intermediate products in the synthesis of antibiotics and dyes. 2- Aminothiazole derivatives are widely used in pharmacology [15]. Some substituted aminothiazole derivatives are used as antioxidant additives to hydrocarbon fuels, minerals and synthetic lubricating oils, solid parafin, polyolefins and vegetable fats. Sym triazine derivatives containing substituents with 2-aminothiazole fragments are effective anticorrosive, antiwear and antiscuff additives to lubricating oils. In continuation of our work 7-14 on the metal complexes of Schiff bases, we report here the study of Schiff base metal complexes of Mn(II), Fe(II), Co(II), Ni(II) and Cu(II) derived from 2-amino-4-phenylthiazole and substituted 4-acetyl-1-phenyl-3-methyl-2-pyrazolin-5-one. Preparation, characterization and antibacterial activity of above metal complexes with Schiff bases Ligand-L and Ligand-L1 are also reported here [16].
The synthesis of unsymmetrical Schiff base has attracted more interest. Although unsymmetrical ligands can clearly offer many advantages over their symmetrical counter parts in the elucidation of the composition and geometry metal ion binding sites in metalloproteins, and in the development leading to the duplication of enzymatic efficiency and selectivity of natural systems with synthetic materials, the difficulty of preparation of such ligands has hampered progress because simple condensation methodology with three components is no longer applied. Meng and coworkers reported that the selective condensation of single -NH2 group might be carried out by strictly controlling reaction conditions and changing the mole ratios of reactants. The chemistry of metal complexes containing salen-type base ligands drived from condensation of aldehyde and amines is of enduring significance. Since they have common features with metalloporphyrins with respect to their electronic structure and catalytic activities that mimic enzymatic oxidation In this paper, we report the synthesis, structural characterization and biological activities of Cu(II), Zn(II), Ni(II) and Mn(II) complexes of the new Schiff base ligand synthesised from 2-hydroxy-1- naphthaldehyde and 5-amino-1-naphthol. The new complexes were investigated by IR, electronic spectra, cyclic voltammetry, conductivity measurement, EPR studies and biological activity [17].
Isatin has been known for about 150 years and has been recently found, like oxindole and endogenous polyfunctional heterocyclic compounds, to exhibit biological activity in mammals. Isatin also is a synthetically versatile substrate that can be used to prepare a large variety of heterocyclic compounds, such as indoles and quinolines, and as a raw material for drug synthesis. Isatin is further known to be a color reagent for the amino acid proline, forming a blue derivative. This property has been exploited for the determination of this amino acid in pollens and other vegetable materials using paper chromatography or for the detection of polymer-bound compounds possessing proline residues. Some isatin derivatives exhibit antiplasmodial activity. Schiff bases and Mannich bases of isatin are known to possess a wide range of pharmacological properties including antibacterial, anticonvulsant, anti-HIV, antifungal and antiviral activity. Bis-Schiff bases are characterized by their capacity to completely co-ordinate a metal ion, forming chelate rings. The Schiff bases of isatin have also been used as ligands for complexation of metals such as copper II. These complexes catalyzed the oxidation of carbohydrates. Bis-Schiff bases can act as inhibitors of human α-thrombin [18]. Recently it has been reported that a bis-imine of isatin has antimicrobial properties and affects cell viability. Here we report the synthesis and characterization of new bis-Schiff bases of isatin, benzylisatin and 5- fluoroisatin, which could be considered as potential biologically active compounds. In the present study we have reviewed the synthesis and different biological activities of some Schiff bases of imidazo-1, 3, 4-thiadiazole derivatives. The Schiff base is functional group that contains a carbon nitrogen double bond with nitrogen atom connected to an aryl or alkyl group but not hydrogen. Schiff bases can be synthesized from a substituted aromatic amines and a carbonyl compound by nucleophillic addition forming a hemiaminal followed by dehydration to generate an imine. The synthesis part starts with the condensation of 1, 3- benzdioxole-5-carboxylic acid and thiosemicarbazide in the presence of POCl3 under reflux to form thiadiazole derivative, various thiadiazoles are further condensed with phenacyl bromide to obtain imidazo thiadiazole derivatives which on treatment with DMF and POCl3 gives 5-formyl derivatives. The formyl functional group has been utilized to synthesize corresponding Schiff bases. The purity of derivatives has been characterized by using IR, NMR and Mass spectra. The substituted derivative show moderate biological activity. Further the prepared Schiff bases have been subjected to antimicrobial property. The derivative has shown moderate to good activity when compared with standard antibiotic ampicillin. Schiff bases have different biological activities such as antimicrobial [19].
Thiadiazole and its derivatives are used for biological activities such as antiviral, antibacterial, antifungal and antitubercular 10. The antileukemic action and host toxicity of the thiadiazoles were blocked by administration of nicotinamide. 1, 3, 4-Thiadiazole are diversified biocidal activities probably by virtue of a toxophoric N=C-S-Grouping. A large number of 4-thiazolidinones have been reported to be antifungal, antibacterial and antileukemic properties. These observations prompted us to synthesis the title compound with a presumption that incorporation thiadizole and thiazolidinones wound produce new compound with significant fungicidal properties. A series of 2-aryl-5-hydrizino-1, 3, 4-thiadiazole exemplified by the structure designed as analogue of the known vasodilator hydrazine and pyridazinyl hydrazine. Subsequent evaluation of this series showed that some analogue possessed both antihypertensive activity and anticonvulsant activity. Furthermore it found that particular substitution in the 2- position of aromatic ring to produced compound reduced with antihypertensive activity with desirable anticonvulsant activity. It was found that methylation of the α-nitrogen of the hydrazine group in the o-tolyl series decrease vasodilator activity without concurrent decrease in anticonvulsant activity [20].
Schiff bases are important class of compounds due to their flexibility, structural similarities with natural biological substances and also due to the presence of imine moiety (-N=CH-) which is potential in elucidating the mechanism of transformation reaction in biological system. These novel compounds could also act as valuable ligands whose biological activity has been shown to increase on complexation. Because of the fundamental and technological reasons, considerable interest has been shown in the synthesis and study of organic solids and metal complexes which behave like semiconducting materials. The electrical properties of the organic ligands and their metal complexes have been intensively studied by many of the research groups all over the world. Metal complexes of the Schiff bases derived from reaction of 2-methylbenzopyrrole-3-carboxaldehyde with some aniline derivatives showing slight semiconducting behavior have been reported. Their conductivity and activation energy were found to depend on molecular structure as well as the ionic radii of the metal ions. Singh measured the electrical conductivity of the complex salts and the heterobimetallic coordination polymers bis (1-ethoxycarbonyl-1-caynoethelene-2,2-dithiolato) cuprate (II) ion and [MM′(cdc)2], [M=Zn(II), Cd(II), Hg(II); M′=Ni (II) or Cu(II); cdc2=cyanodithioimidocarbonate]. All the complexes exhibited semiconducting behavior. Semiconducting properties of Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and VO(IV) complexes of Schiff base derived from resdiacetophenone and S-benzyldithiocarbazate were studied by Makode. It was found that complexes are semiconducting in nature [21].
Schiff bases are typically formed by the condensation of a primary amine and an aldehyde. Schiff bases are important intermediates for the synthesis of various bioactive compounds. Furthermore, they are reported to show a variety of biological activities including antibacterial, antifungal, anti cancer and herbicidal activities. On the other hand, they are fundamental material for synthesis of various Schiff base ligands which used as chiral auxiliaries in asymmetric synthesis. Metal complex Schiff bases have also been used in oxidation reactions. In view of these facts we can clear about that Schiff base are important not only in medical chemistry, but also in organic synthetic chemistry. Schiff base perhaps are synthesized in various method. In this paper, we will research which the simple way to synthesize Schiff base via compare of three primary methods. For the sake of convenience to compare the result, we choose the simple material (3, 4, 5-trimethoxybenzaldehyde and p-toluidine) to synthesize simple Schiff base [22].
Amino heterocyclic compounds containig two or more potential donor centers play an important role in the study of competitive reactivity of an bidentate ligand system. Heterocyclic phenazone and their derivatives (4-amino antipyrine) are know to act as bidentate or tridentate ligands when coordinated to metal ion. Schiff base of 4-amino antipyrine and its complexes are know for their variety of application i.e., in the areas of catalysis clinical application and pharmacologically. Also the chemistry of antipyrine and its derivatives has been extensively investigated due to its physiological properties. The study of the metal complexes of antipyrine in antineoplastic medication, molecular biology and bio engineering has become hotspots in recent years. This work describle the preparation new Schiff base and its some complexes derived from 4-amino antipyrine, sulphadiazine and acetoacetanilide. The ligand is found to be chelating with metal ion in neutral tridentate manner through the azo methane nitrogen atoms and oxygen group of the acetoacetanilide.
Pyridine derivatives have been of great interest because of their role in natural and synthetic organic chemistry. Many products which contain a pyridine subunit exhibit biological activity such as antimicrobial and antituberculosis activities. So the pyridine containing Schiff bases are expected to have enhanced biological activities. It is well established that the biological activity associated with the hydrazone compound attributed to the presence of the active pharmacophore (-CONH-N=C-). Hence many hydrazone compounds containing this active moiety showed good biological activities according to the literature. In the present work, we have synthesized fifteen Schiff base from nicotino hydrazide with substituted aromatic benzaldehydes afforded title compounds (2a-n) and evaluated their in vitro antioxidant activity.
Schiff bases, products of the reaction of primary amines and carbonyl compounds, are involved in many metabolic processes. Numerous products of further fragmentation and crosslinking are responsible for the color, flavor, and taste of foods and drinks. 1 Salicyliden- and 2-hydroxynaphthylideneamines have been the subject of particular interest because some of their complexes are found in nature and biological activities have been recorded for the synthesized ones. Pyrimidine is the parent heterocycle of a very important group of compounds that have been extensively studied due to their occurrence in living systems. Pyrimidines are reported to have a broad spectrum of biological activities. Some are endowed with antitumor, antiviral, antiinflammatory, antipyretic, antimicrobial, and antifungal properties. Considerable attention has been given to the metal (II) complexes of polydentate Schiff base ligands of the N-aminopyrimidine type, due to their structural richness and electrochemical properties as well as their potential as a model for a number of important biological systems. This paper describes the synthesis of a new Schiff base ligand containing a ring of pyrimidine and its metal complexes. Spectral and magnetic studies were used to characterize the structure of the complexes. IR, 1 H-NMR, 13C-NMR, and mass spectra were obtained to determine the structure of the ligand (HL). All of the synthesized compounds were evaluated for their antimicrobial activities against gram-positive and gram negative bacteria and fungi using the microdilution procedure.
The synthesis of high nitrogen containing heterocyclic systems has been attracting increasing interest over the past decade because of their utility in various applications, such as propellants, explosives, pyrotechnics and especially chemotherapy. In the medicinal chemistry, azoles are widely used and studied class of antimicrobial agents due to their safety profile and high therapeutic index. Among these, conazoles are a major class of azole-based drugs such as itraconazole, fluconazole, voriconazole, ravuconazole etc. Some of other major applications of conazoles are on crop protection. As pharmaceuticals, they are used for the treatment of local and systemic fungal infections, which are important problems in phytopathology and especially in medicine, and they are frequently observed in immune-compromised patients suffering from AIDS or subjected to invasive surgery, anti-cancer therapy or graft receivers. Several lines of evidence suggest that the primary target of azoles is the heme protein, which cocatalyzes cytochrome P-450-dependent 14α-demethylation of lanosterol. Inhibition of 14-demethylase leads to depletion of ergosterol and accumulation of sterol precursors, including 14-methylated sterols (lanosterol, 4, 14- dimethylzymosterol, and 24-methylenedihydrolanosterol), resulting in the formation of a plasma membrane with altered structure and function. The more recent triazole derivatives, such as fluconazole, itraconazole, and voriconazole owe their antifungal activity at least in part to inhibition of cytochrome P-450-dependent 14α-sterol demethylase. The diseases caused by fungal species cause not only improve the cost of therapy but also may lead mortality. Due to the inadequacy of alone standard antibiotic therapy in certain circumstances, more efforts have been focused on addressing the problem of multidrug-resistant bacteria and the decreasing of costs and consequences the obtained results from this. Tuberculosis (TB) that is another mortal infection, causes to death with approximately three million patients in the world every year. According to the World Health Organization (WHO), about 30 million people will be infected within next 20 years. Thus, the treatment of infections has become an important and challenging problem because of the increasing number of multi-drug resistant microbial pathogen. In spite of a large number of antibiotics and chemotherapeutics available for medical usage, the increasing resistance made it necessary to continue the search for new antimicrobial substances. Though various molecules designed and synthesized for this aim, the efforts have demonstrated that 1, 2, 4-triazoles and their derivatives could be considered as possible antimicrobial agents, some of them studied in our laboratories In the present study, as a continuation of their studies on obtaining bioactive molecules, researchers have performed the synthesis of some new 1, 2, 4-triazole derivatives and investigation of antimicrobial activities of newly synthesized compound.
Azo dyes constitute one of the largest and most varied groups of synthetic organic dyes in use today. Azo compounds are highly important, wellknown and widely used substances in the textile, paper, coloring agents for foods and cosmetics industries. Other applications include emerging technologies like liquid crystals, organic photoconductors and non-linear optics. Azo compounds serve as important analytical tools by providing a strongly chromophoric label, the concentration of which is easily determined by colorimetric, spectrophotometric or spectrofluorimetric methods. Besides, azo compounds are important analytical aid compounds serving as pH indicators, complexometric indicators and to a lesser extent, pre-concentration reagents. The pharmacological use of azo compounds originates from the discovery of the antibacterial action of prontosil on streptococcal infections by Dogmagk.
Synthesis of different substituted Schiff bases carbazates
The scientists were synthesized by reacting [Cu(Sac)2(H2O)4]2H2O with the appropriate ligands in EtOH new mixed ligand complexes [Cu(NNS)(sac))] (NNS1=S-benzyl-β-N-(2-acetylpyrid-2-yl) methyleneditiocarbazate, NNSIV=S-benzyl-β-N-(2- benzoylpyrid-2-yl) methylenedithiocarbazate and NNSII=S-benzyl-β-N-(6-methylpyrid-2-yl) methylenedithio-carbazate, sac= the saccharinate anion). Magnetic and spectral evidence indicate that the complexes are four-coordinate in which the Schiff bases coordinate as NNS ligands and the sac anion coordinates as a unidentate N donor ligand. An X-ray crystallographic structural analyses of [Cu(NNS1) (sac)] shows that the complex has a distorted square-planar geometry with the Schiff base coordination to the Cu (II) ion as a uninegatively charged. Tridentate chelating agent via the pyridine N atom, the azomethine N atom and thiolate S atom while the 4th coordination position is occupied by the N-bonded saccharinate anion. [Cu (qaldsme) (X) (MeOH) n] and [Ni (qald sme) 2] 0, 5 MeCN (qaldsme=anionic form of the 2-quinoline carboxaldehyde Schiff base of Smethylditiocarbazate; X=NCS, J-, NO3-; n=0 or (1) were synthesized and characterized by magnetic and spectroscopic techiniques. X-ray crystal structure determined of [Cu(qaldsme) (ONO2) (MeOH)], (1) [Cu(qaldsme) (NCS)] (2) and [Ni(qaldsme) 2]-0,5 MeCN (4) shows the nitrato complex of Cu (1) is monomeric and five coordinate and the thiocyanotocomplex has a novel thilate S-bridged dimeric structure in which each of the Cu(II) ions adopts a five coordinate approximately square-pyramidal geometry with a CuN3S2 coordination Kernel. The Ni (II) complex has a diistroted octahedral geometry with meridional disposition of the two uninnegatively charged tridentate NNS ligands. In all these complexes, the Schiff bases are coordinated in their iminothiolate forms via the quonoline N atom, the azomethine N atom and the thiolate S atom. New Sn (IV) complexes of empirical formula, Sn (NNS)I3 (NNS=anionic forms of the 2-quinolinecarboxaldehyde Schiff bases of –methyl-and S-benzyldithiocarbazate were prepared and characterized by a variety of physicochemtechniques. In the solid state, the Schiff bases exist as the thionate tautomer but it solution and in the presence of Sn(IV) iodide they convert to the thiol tautomer and coordinate to the Sn atom in their deprotonated thiolate forms. The structures of the free ligand, H qaldsbz and its trriiodotin (IV) complex? [Sn(qaldsbz)I3] were determined by X-ray diffraction.
Two new pyrimidine based NNS tridentate Schiff base ligands S-methyl-3-((2-S-methyl-6-methyl-4- pyrimidyl)methyl)dithiocarbazate [HL1] and S-benzyl-3-((2-S-methyl-6-methyl-4-pyrimidyl)methyl) dithiocarbazate [HL2] were synthesized by the 1:1 condensation of 2-S-methylmercapto-6-methylpyrimidine-4-carbaldehyde and S-methyl/S-benzyl dithiocarbazate. A Ni(II) complex of HL1 and Co(II) and Fe(III) complexes of HL1 were prepared and characterized by elemental analyses, molar conductivities, magnetic susceptibilities and spectroscopic studies. All the bis-chelate complexes have a distorted octahedral arrangement with an N4S2 chromophore around the central metal ion. Each ligand mol binds the metal ion using the pyrimidyl and azomethine N and tholate S atoms (except in the Ni complex, one ligand mol uses the thione S in lieu of thiolato S atom). In the Ni(II) complex, one of the ligand mols behaves as a neutral tridentate and the other mol functions as a mononeg tridentate, whereas in the Co(III) and Fe(III) complexes, the ligand mols behave as monoanionic tridentate. All the complexes were anakysed by single crystal x-ray diffraction and significant differences concerning the distortion from an octahedral geometry of the coordination environment were observed.
New bis-chelated Zn?? and Cd?? complexes, [M(mpsme)2(mpsme=the anionic form of the tridentate ONS donor ligand formed from Me pyruvate and S-methyldithiocarbazate) were prepared and characterized by conductance. IR, electronic and NMR spectroscopic techniques. Spectral evidence supports a six-coordinate distorted octahedral structure for these complexes. X-ray crystallography structural analyses also confirms that, in both the [Zn(mpsme)2 and [Cd(mpsme)2] complexes, the Me pyruvate Schiff base of S-methyldithiocarbazate is coordinated to the metal ions as a uninegatively charged tridentate ONS chelating agent via the carbonyl O atom, the azomethine N atom and the thiolate S atom. Both complexes are assigned a distorted octahedral geometry in which the ligands are arranged meridionally around the metal ions. The distortion from regular octahedral geometryis attributable to the restricted bite angles of the ligand.
Condensation of SMDTC with 2-furyl-methylketone and 5-methyl-2-furaldehyde gave isomeric Schiff bases, (NS) and (NS1). The metal complexes of these uninegatively charged bidentate Schif base ligands with Cd, Sn, Fe, Pb, and Co were repared. The complexes were characterized by a variety of physicochemical techniques. X-ray crystallographic analyses shows that the Cd(II) complex, bis [S-methyl-β-N-(2-furylmethylketone) dithiocarbazato] cadmium (II), consists of two mols with distorted octahedral structure. The Co(II) complexes are paramagnetic with a square-planar stereochemical. The diamagnetic Sn(II), Fe(III) and Pb(II) complexes also have square-planar structures while the diamagnetic [Cd(NS1)2] 3 H2O complex is tetrahedraly. The [Cd(NS1)2 3H2O, Sn(NS)2 and [Co(NS)2] showed clear inhibition of almost all bacteria and fungi tested. However, [Cd(NS)2], [Fe(NS)] Cl2, [Pb(NS)2] and [Pb(NS1)2 were inactive against all bacteria assayed while [Cd(NS)2], [Fe(NS)] Cl2, [Pb(NS)2] are very active against human cell T-lymphoblastic leukemia (CEM-SS) and cervical cancer cell (HELA) with CD50 values between 1.8 and 3.6 μL cm-3.. Isomeric bidentate ligands having N-S donor sequence were prepared by condensing S-Benzyldithiocarbazate (SBTC) with 5- methyl-2-furylaldehyde (NS) and 2-furyl Me ketone (NS1). [ML2] (M=Pb, Fe, and Cd) and [ML2] Cln (M=Sn, n=2 and Co, n=1) (HL=NS and NS1) were prepared. The compounds were characterized by spectroscopic studies (IR, 1H NMR and electronic spectra). X-ray crysatllographical analyses of S-benzyl-β-N-(5-methyl-2-furylmethylene) dithiocarbazat shows two independent mols in the asymmetric unit. The mol adopts a trans-cis configuration, as was obsorbed in other analogs, such as SBDTC where the furylmethylene and benzyl groups are trans and cis about the N-C and C-S bonds, responsibility. The mol structure of bis[S-benzyl-β-N-(2-furylmethhylene) dithiocarbazato] cadmium (II) shows a tetrahedral geometry about the central Cd atom with the bidentate ligand coordinating through the thioketo S and the azomethine N atoms. The Pb(II) complex of the NS ligand was highly cytotoxic against leukemic cells (CEM-SS) with a CD50 of 3.25 μg cm-3 while antimicrobial screening showed that the [Fe(NS)2]Cl2H2O complex was effective against Aspergillus achraceous.
Two new isomeric Schiff bases, S-methyl-β-N-(2-furylmethyl)methylenedithiocarbazate (NS1) and S-methyl-β-N-(5-methyl-2- furyl)methyleneditiocarbazate (NS11) were prepared. Bis-chelated complexes of these two bidentate ligands, [M(NS)2], [M=Cu, Ni and Zn], were synthesized. The Schiff bases and their metal complexes were characterized by a variety of physicochemical techniques. X-ray crystallography analyses shows that [Zn(NS1)2 is four-coordinate and has a distorted tetrahedral structure with the ligand coordinated to the Zn(II) ion as an uninegatively charged bidentate chelating agent via the azomethine N and the mercaptide S atoms. The Cu(II) complexes are paramagnetic with a square-planar stereochemistry. The Ni(II) and [Zn(NS11)2] complexes have a square-planar and tetrahedral structure, responsibility, however, they are diamagnetic. Only Cu(NS1)2 showed clear activity against the bacteria, Subtilis mutant (B28), while both NS1 and NS11 Schiff bases were strongly antifungal against Saccharomyces cerevisae (20341). Candida albicans, Candida lipolytica (2075) responsibility. The Cu(NS1)2, Ni(NS1)2 and Zn(NS1)2 complexes showed very good activity against human cell T-lymphoblastic leukemia (CEM-SS) cells with CD50 values of 1.6 μg ml-1, 2.1 μg ml-1 and 3.0 μg ml-1, responsible. The remainder of the Schiff bases and complexes were inactive towards CEM-SS cells. None of the compounds showed any activity towards colon cancer cells (HT-29). Only the Cu(NS1)2 and Zn(NS1)2 complexes were highly active against cervical cancer cells (HELA cells) with CD50 values of 1.5 μg ml-1 and 2.1 μg ml-1, while the Ni(NS1)2 complex was weakly active towards HELA cells with a CD50 value of 23.0 μg ml. Four 5-coordinate (CuLQ)ClO4(HL=s-R-β-N-(pyridine N-oxide-2-ylmethylidene) dithiocarbazate (R=Me, CH2 Ph): Q=o-phenanthroline (phen), 2, 21-bipyridine) were synthesized and characterized by UV, IR spectra and magnetic susceptibilities at room temperature. The xray crystal structure of [Cu (L) phen]ClO4(R=CH2Ph) was detected. The crystal structure contains a mononuclear unit in which Cu(II) displays a distorted square pyramidal geometry. Crystal data monoclinic, space group C2/c, a 19.227 (5), b 14.506 (3), c 20.487(4), β 106.00 (2), Z=8, R=Rw=0.046.
New organometallic Sn(IV) complexes of the empirical formula Sn(NNS) Ph2Cl (NNS=anionic forms of the 2- quinolinecarboxaldehyde Schiff bases of S-methyl-and S-benzyldithiocarbazate) were prepared and characterized by IR, electronic, 1H NMR and ES mass spectroscopic techniques. The molecular structures of the 2-quinolinecarboxaldehyde Schiff base of S-methyldithiocarbazate (Hqaldsme) and its diphenyl tin (IV) complex. Sn(qaldsme)Ph2Cl, were detected, by x-ray diffraction. In the solid state, the ligand remains as the thione tautomer in which the dithiocarbazate chain adopts an E, E configuration and is almost coplanar with the quinolone ring. The Sn(qaldsme)Ph2Cl complex crystallizes in two distinctly different conformationally isomeric forms, each having the same space group but different lattice parameters. X-ray analyses as a uninegatively charged tridentate chelating agent via the quinolone N atom, the azomethine N atom and the thiolate S atom. The two Ph groups occupy axial positions and the chloride ligand occupies the 6th coordination position of the Sn atom. The deprotonated ligand adopts an E, E, Z configuration in the complex.
New mixed-ligand complexes of general empirical formula, [Cu(NNS)(sac)(H2O)] (NNS1=S-methyl-β-N-(6-methylpyrid-2- yl)methylenedithiocarbazate.
NNS11=S-methyl-β-N-[(2-pyridyl) phenylmethylene] dithiocarbazate, sac=saccharinatye anion) were synthesized by reacting [Cu(sac)2(H2O)4]2H2O with the appropriate ligands in water-EtOH mixters and characterized by elemental analyses and conductance, magnetic, IR and electronic spectroscopic measurements. Magnetic and spectral evidence support a five-cordinate geometry for the complexes in which the Schiff bases coordinate as NNS tridentate ligands and the saccharinate anion coordinate as a unidentate N-donor ligand. An x-ray crystallographic structural analyses of [Cu (NNS1)(sac)(H2O)] shows that thecomplex has a distorted square-pyramidal structure in which the Schiff base is coordinated to the Cu ion as a tridetate NnNS chelating agent via the pyridine N atom, the azomethine N atom and the thiolate S atom, the 4th and 5th coordination positions of the fivecoordinate Cu(II) ion being occupied by the imino N of the saccharinate anion and O atom of the aqua ligand. The complexes were evaluated for their biological activities against eight pathogenic microbials and human T-lymphoblastic leukemia cell lines. The complexes exhibit marked cytotoxicity against leukemic cell lines and display moderate activity against pathogenic bacteria and fungi.
New mixed-ligand Cu(II) complexes of empirical formula [Cu(pysme)(sac)(MeOH)] and [Cu(6mptsc)(sac)]2 were synthesized and characterized by conductance, magnetic, IR and electronic spectroscopic techiniques. X-ray crystallographical structure analyses of these complexes indicate that in both complexes the Cu(II) ions adopt a five-coordinate distorted square-pyramidal geometry with an N3SO donor environment. The Schiff bases are coordinated to the Cu(II) ions as tridentate NNS chelates via the pyridine N atom, the azomethine N atom and the thiolate S atom. In the monomeric [Cu(pysme)(sac)(MEOH)] complex, the saccharinate anion acts as a monodentate ligand coordinating the Cu (II) ion via the imino N atom whereas in the dimeric [Cu(6mptsc)(sac)]2 complex, the sac anion behaves as a bridging bidentate ligand providing the imino N donor atom to one of the Cu(II) ions and the carbonyl O as a weakly coordinated axial ligand providing the imino N donor atom to one of the Cu(II) ions and the carbonyl O as a weakly coordinated axial ligand atom to the other Cu(II) ion. In both complexes the Cu(II) ions have distorted square-pyramidal environments. The distortion from an ideal square-pyramidal geometry is atributed to the restricted bite angels of the planar tridentate ligand.
Condensation of SMDTC with 2-furyl-methylketone and 5-methyl-2-furaldehyde gave isomeric Schiff bases, (NS) and (NS1). The metal complexes of these uninegatively charged bidentate Schiff base ligands with Cd, Sn, Fe, Pb and Co were prepared. The complexes were characterized by a variety of physicochemical techiniques. X-ray crystallographic analyses shows that the Cd(II) complex, bis[S-methyl-β-N-(2-furylmethylketone) dithiocarbazato] cadmium (II), consists of two molar with distorted octahedral structure. The Co(II) complexes are paramagnetic with a square-planar stereochemistry. The diamagnetic Sn(II), Fe(III), and Pb(II) complexes also have square-planar while the diamagnetic [Cd(NS1)2]3 H2O complex is tetrahedral. The [Cd(NS1)2]3H2O, [Sn(NS)2] and [Co(NS)2] showed clear inhibition of almost all bacteria and fungi tested. However, [Cd(NS)2], [Fe(NS)]Cl2 and [Pb(NS)2] showed clear inhibition of some fungi. The [Cd(NS)2], [Cd(NS1)2]3H2O and [Co(NS)2] are very active against human cell T-lymphoblastic leukemia (CEM-SS) and cervical cancer cells (HELA) with CD50 values between 1.8 μL cm-3 and 3.6 μL cm-3.
A new dithiocarbazate ligand, S-2-picolydithiocarbazate (S2PDTC) was synthesized using 2-picolyl chloride hydrochloride. Tridentate Schiff base were prepared by condensation of S-2-picolyldithiocarbazate (S2 PDTC) with pyridine-2-carboxaldehyde (NNS1), 2-acetylpyrrole (NNS11) and 2-acetylthiophene (NSS), while a bidentate Schiff base (NS) was prepared by condensing the S2 PDTC with 2-acetylfuran. Complexes of S2 PDTC and its Schiff bases with Ni(II) salts were synthesized and characterized by elemental analyses and various physicochemical techniques. A square-planar structure is proposed for the diamagnetic [Ni(S2PDTC)2 and [Ni(NSS)2] complexes while [Ni(NS)Cl] complexes was dimeric. All of the Ni(II) complexes were inactive against CEM-SS cancer cells.
Hydrogen bonded Schiff Bases
In the next work describes synthesis of hydrogen-bonded pseudo-dimer, [Mn(III) L1(CH3CH2OH]2(ClO(4))(1)(L1=N, N1-bis(2- hydroxy-1-napthalidenato)-1, 2-diaminopropane) has been synthesized. The single crystal X-ray diffraction reveals that the structure affords an longated octahedral MnN (2) O (4) coordination environment, geometry with the four donor atoms of the tetradentate Schiff base in the equatorial plane and with two ethanol molecule in axial positions with Mn-O=2.265 (2) and 2.266 (2) AA.
In this research we report on the study of hydrogen bonds of the Schiff base and water molecules in D85 S in the absence and presence of various halides, assigning their N-D and O-D stretching vibrations in D2O, respectively in low-temperature Fourier Transfer in Infrared (FTIR) spectroscopy. They found that the hydrogen bond of the Schiff base in D85S (Cl (-)) is much stronger than that in HR, being as strong as that in wild-type BR.
The next work describes the synthesis of Schiff bases containing pyridoxal (PL), N- (pyridoxylidene-tolylamine, C(15) H(16) N(2) O(2) (I), N-(pyridoxylidene)-methylamine C(9) H(12) N(2) O(2) (III) and their 1:1 adduct with 2-nitrobenzoilacid, (I) (+) C(7) H(4) NO4:- (II) and 4-nitrobenzoil acid (III) (+) C(7) H(4) NO4: (IV) serve as models for the coenzyme pyridoxal 51- phosphate (PLP) in its PLP-dependent enzymes. These models allow the study of the intramolecular OHN hydrogen bond of PL/PLP. Schiff bases and the H-acceptor properties of their rings. The proton in the intramolecular O-H...N hydrogen bond of (I)/ (III) is located close to oxygen (enolamineform). In this work describes low temperature Fourier Transform Infrared (FTIR) spectroscopy to the all-trans form of ASK at 77 K, and compared the local structure around the chromophore and their structural changes upon retinal photoizomerization with those of BR. Scientist determined that the weak hydrogen bond of the bridged water between the Schiff base and Asp 75 originates from their geometry.
The synthesis, and structural characterization of seven heterodinuclear complexes (1); [CuII(HL)Na1(NO3)(MeOH)](1), [Cu?? (HL)Pb??(NO3)2] and [Cu??(HL)Cd??(NO3)2] (7) are reported, where H3L=9=N, N1-bis (3-methoxysalicylaldiimine)-1, 3- propylene-2-ol. Compounds 1 and 3 crystallize in the monoclinic P2, /h space group, 4, 5 and 7 in the monoclinic P2, /c space group, while the space group of complexes 2 and 6 is triclinic P1. The X-ray crystallography, reveals that the structures of all the complexes consist of dephenoxo-bridged heterometallic cores in which Cu?? metal ion is trapped in N2O3 compartment of the Schiff base ligand ühile the second metal ions present in the larger and open O4 [O(phenoxo)2O(metoxy)2] compartment.
Polymeric Schiff bases [Cu3(TFSSB)2(H2O)4 4H2O]n (TFSSB=taurine 3-formylsalicylic acid Schiff) was synthesized from TFSSB and Cu(II) acetate monohydrate in EtOH solution and the crystal structure was detected by x-ray diffraction method. The crystal belongs to monoclinic system, space group P21/n, with a=0,9279 (6) nm, b=1.1730 (2) nm, c 1.471 (2) nm, β 106.96 (2), V=1.531 (2). Z=2, dc=1.890 cm-3, μ=2.291 mm-1, F (000)=882, R1=0.0259, wR2=0.0659. The Cu 1 is five-coordinate, the Cu2 is four-coordinate.
Two new Schiff base macrocycles-a 4+4 condensation product and a meso-type 2+2 condensation product-were obtained in a reaction of trans-1,2-diaminocyclohexane and 2,6-diformylpyridine. Reduction of these compounds led to the corresponding 4+4 and 2+2 macrocyclic amines. The macrocycles were characterized by NMR spectroscopy and electrospray mass spectrometry. The symmetry and streochemical of these macrocycles, as well as of new 3+3 and 4+4 diastereomers identified in solution, has been established. X-Ray structures of the 2+2 and 4+4 Schiff basemacrocycles confirm the configurations detected on the basis of spectroscopic investigations. The crysat structure reveal that the centers of the square-shaped 4+4 macrocycles from channels as a resut of columnar stacking.
Distribution of the electric charge in electro- and photoactive polymers poly [Msalen] and poly [MSalbn] (M=Cu(II), Ni(II), Pd(II), Salen=bis(salicylidene)ethylenediamine: Salbn=1, 4-bis(salicylidene-1,4-butylenediamine) was studied using XPS. The results of comparative analyses of the binding energy of the inner 1 s-electrons of functional N and O atoms were discussed. Distribution of electron d in free ligands, in complex monomers and cossesponding polymers ere also discussed. Intramolecular charge transport and electric conductor of a structural unit of poly [MSalen] were discussed. New series of macrocyclic Schiff base lanthanide (III), Y(III) and Cd(II) complexes ç[M(1)]Xn(X=NO3-, M=Y, Ln=La-Yb except Pm and Dy; X=ClO4-, M=Cd, La, Ce, Pr, Sm, Gd, or Er) and [M(3)] Xn(X=NO3-, M=Dy: X=ClO4-, M=Er and Cd), were prepared by cyclocondensation of O1, O7-bis (formylphenyl)-1,4,7-trioxahptane (1) Or tris(2-aminoethyl)amine (3) in the presence of the appropriate metal salt as a template agent. The Schiff-base macrocycles 1 and 3 are also formed in the absence of a metal ion. Treatment of 1 with NaBH4 in MeOH gives the diamine macrocycle 2. The reactions of Ln??? Cd???ç and Y??? ions üith 2 also üere studied. The crystal structures of monoprotonated 2 and of [Cd(3)] (ClO4)2 were detected by x-ray diffraction analyses.
Furan-2, 5-dicarboxaldehyde with [H2NXO(CH2)2]2O [X=1, 2-C6H4, (CH2)2] in EtOH or BuOH in the presence of MSCN (M=Ca, Sr, Ba) gave the corresponding macrocycles. The structure of I [X=(CH2)2, M=Sr] was detected by x-ray crystallography. A new di-Schiff base ligand was prepared from 3-acetylpyridine and 1, 2-diaminoethane and its structure also was characterized by single crystal x-ray diffraction. Also, the ligand formsa 1:1 comlex with Ag(NO3) in MeCN. The crystal structure of the complex exhibits a 1-dimensional-double helix with the pitch length of 10 A. In these reactions, the ligand was found to disintegrate into the individual components to form a crystal of a 3-dimensional-network which comprised of Ag(NO3) and 1, 2- diaminoethane. Nitrate ions occupied the channels and form several NOH bonds with the wals the channels. The topology of the 3-dimensional-network is similar to that of the CdSO4. This structure prompted the authors to study the reactions of Ag(NO3) with 1, 2-diaminoethane, 1, 2-diaminopropane ans 1, 3-diaminopropane. The crystal structures of these complexes reveal that 1, 2-diaminopropane and 1, 3-diaminopropane and 1-dimensional-zigzag chain, structure. Different application of Schiff bases.
DNA binding agents
Nowadays we know that, there is no possibility recovering all of desease without binding DNA. Some of complexes Schiff bases show DNA binding properties they can bind DNA through intercalation with the binding consentration at the order of different magnitude. In this work scientists have been synthesized new Ho complexes of Schiff-base ligands derived from 8- hydroxyquionoline-2-carboxyaldehyde and aroylhydrazines. Studing of DNA binding properties showed that all the ligands and Ho(III) complexes can bind to Calf thymus DNA through intercalation with the binding const. at the order of magnitude 105 M-1- 106 M-1, but complexes present stronger affinities to DNA than ligands. It has been written that all complexes may be used as potential anticancer drugs.
It has been reported synthesis of new N, N1-bis(3, 5-tret-butylsalicylidene-2-hydroxy)-1,3-propanendiamine substituted binuclear Cu(II) complexes. All synthesized complexes can cleave plasmid DNA to nicked DNA in a sequential manner as the concentration or reaction times are increased in the absence of reducing agent. Their cleavage activities are promoted in the presence of H2O2. They showed that cleavage mechanisms between the complexes and plasmid DNA probably involve singlet oxygen 1O2 and OH as reactive oxygen species used. Next work also reported binuclear Cu(II) complexes derived from salicyaldehyde and 2-mercaptoethylamine. Investigation shows that the complexes behaiving photo-induced cleavage of supercoiled pUC 19 DNA in UV light of 365 nm and red light of 633 nm (He-Ne laser). The DNA photocleavage reaction involves formation of singlet oxygen (1O2) as the reactive species in a type-II pathway. In this work described the synthesis of oxovanadium complexes derived from N, N1-dimetylenediamine and 0-hydroxy-acetophenone. Scientist also investigated DNA binding ability and photoinduced DNA cleavage activity. The complex binds to double-stranded DNA giving a Kaa value of 1.56 × 107 M-1 and displays DNA cleavage activity on UV (300 nm) irradiation via a mechanistic pathway involving formation of singlet oxygen as the reactive species. In other works, shown synthesis of Cd(II) complexes derived from 2, 6-bis [1-94-amino- 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxopyrimidin-5-yl-imino]ethylpyridine. After investigation electrophoretic experiments indicate that the Cd complex induces cleavage of the plasmid pBR322 DNA to give ulterior nicking and shortening of this mol. AS a result of the complex binding to DNA, resulting in the conclusion that 1 behaves as a chemical. Nuclease. Cytotoxic activity of the Cd(II) complex against selected different human cancer cell lines is specific and increases with increasing concn medium is supplemented with 1 a remarkable inhibition of the growing cell is obsd, important cell degeneration appears before 48 h and abundant ppts. Are formed that correspond to cell residues and denatured proteins. Next work investigate the synthesis of new ternary Cu(II) complexes derived condensation of 2-mercaptoethylamine hydrochloride with salicylaldehyde or 2-hydroxy-3- metoxybenzaldehyde. Complexes show DNA cleavage activity. The complexes exhibit quasireversible cyclic voltammetric respose in DMF-Tris buffer for the Cu(II)/Cu(I) couple. New Copper (II) of different nuclearities viz [Cu(salmet)(bpy)} (1), [Cu (salmet)(Hlm)]2 (2) and [Cu(salmet)(1-Melm)] (3), where salmet is dianionic Schiff base N-salicylidene-L-metioninato, were prepd, structually characterized by x-ray crystallog and their hydrolytic DNA cleavage activity studied. The copper complexes show significant DNA cleavage activity in the dark giving an order 1>3>2. The hydrolytic nature of the DNA cleavage is evidenced from the control expts. Showing no apparent inhibition of cleavage under argon atm. And in the presence of hydroxyl radical inhibitor DMSO or singlet oxygen quencher azide ion.
Antimicrobial properties
In this part of research we have showed the antimicrobial properties of Schiff bases. We are going to describe diffferent scientific research about Schiff bases antimicrobial properties. First research devoted to studing of antimicrobial properties of 5- methylpyrazole-3yl-N-(2-hydroxyphenylamine) methyleimine and its complexes (Co(III), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II)). Researcher have described in vitro antimicrobial activity of ligand and the metal complexes and it have been studied by SEM against some pathogenic bacteria.
The next work were described synthesis and antimicrobial properties of 2-(E)-(4-metoxyphenylimino) phenol and the metal complexes (Mn(II), Co(II), Ni(II), Cu(II) and Zn(II)). The antimicrobial activity investigation shows that M(II) and Zn(II) show against the species Aspergillus niger higher antifungal activity. In this work were studied synthesis, characterization, 2-((E)-(2, 4- dibromophenylimino)methyl)-4-bromphenol and the complexes (Cu(II) and Ni(II). The investigation of antimicrobial properties üere investigated against tüo bacteria (E coli and Salmonella typhr) and tüo fungi (Pencillum, Aspergillus sp). They have been determined that the complexes shoüs more biological activity than the Schiff base. The next work shows synthesis and urease inhibition of Schiff bases derived from isoniazid and fluorinated benzaldehyde and their copper complexes. All synthesized compounds were evaluated for their antimicrobial activity and urease inhibition. All copper (II) complexes showed excellent inhibitory properties against jack bean urease, considerably better than that of the standart acetohydromamic acid. The next report was described synthesis of 2-((E-(2-pyridine-2-pyridin-2-ylthio)ethylimino) methylphenol substituted Co(II) and Ni(II) complexes. Study antimicrobial properties determines that this compounds shoü antibacterial activity as üell as catecholase activity.
Trimethylsilyl-propyl-p-aminobenzoate and its Cu(II), Zn(II) complexes were synthesized. The antifungal and antibacterial properties of the prepared compounds against Aspergillus fumugatus ATCC 66567. Penicillium chrysogenum ATCC 20044, Fusarium ATCC 20327 Baccillus sp ATCC 31073. Pseudomonas sp. ATCC 15780 were evaluated/both Schiff bases and metal complexes showed better antimicrobial activity compared to the standart compounds Caspofungisid and Kanamyan. 2,21-(((azanediyl bis(propane-3,1-diyl))bis(azanylylidene))bis(methanylylidene)dephenol substituted Ni(II), Zn(II), Fe(II) and Cu(II) üas synthesized. Antibacterial activity of ligand and its transition-metal complexes üas studied using disk diffusion assay. Zn(II)-1 and Ni(II)-1 exert a high inhibition of the growth of all bacterial strains with inhibition diameters ranging from 8 mm to 14 mm.
S-allyl-2-(4-benzyloxyphenylmethylene) hydrazinecarbodithioate substituted Ni(II), Cu(II), Zn(II), Cd II), Pd(II) complexes was synthesized. The in vitro bactericidal activity suggests that the palladium (II) complex is strongly active against two bacteria. The cadmium (II) complex is moderately cytotoxic with an LC50 value of 409 μg/ml. The Schiff bases ligand, 1-phenyl-3-methyl-5- hydroxypyrazole-4-metylene-81-quinolineimine and its Cu(II), Zn(II) and Ni(II). The ligand and its metal complexes were subjected to cytotoxic test and the metal complexes show significant cytotoxic activity against lung cancer A 549cell.
Ferromagnetic interaction in Fe
It have been synthesized N,N1-bis(2-hydroxy-1-napthaldehyde)-1,2-phenylendiamine; L2-N,N1-bis(salicylidene)-1,2- phenylendiamine, L3-N,N-bis(5-ll-salicylidene)-1,2-phenylenediamine: 4,41-bpy=4,41 bipyridene and their metal complexes.
Researchers determined that the ferromagnetic interaction obsorbed in second complex in tentatively ascribed to the dimer formation through Fe-π interaction at low temperature. The next work was described the synthesis of five new octahedral Fe(II) complexes [FeL2(4-dpa)In(EtOH) (1), [FeL2(bipy)In(DMF) (2). All compounds are characterized using x-ray structure analyses and T-dependent suspectibility measurement. Both methods indicate that all Fe(II) are in the paramagnetic high-spin state over the whole temperature range studied. The O-Fe-O angle, the so called bit of the equatorial ligand, is with an average of 1110 in the region typical for high spin Fe(II) complexes of this ligand type. In the case of compound 1 and infinite two-dimensional H bond network can be found for the compounds second and fourth H bond interaction are observed between the complexes molecules. A comparison of the curve progession obtained from the magnetic measurements of the mononuclear complex fifth and the polymeric complexes 1-3 indicated that no magnetic interactions are mediated over the bridging axial ligands. For the dinuclear complex 4 weak antiferromagnetic interactions between the two Fe centers are found.
Five binuclear Schiff base Cu(II) complexes [Cu2(L)(OAc)]3DMF (1) [Cu2(L)(OAc)]2 3DMF (2), [Cu2(L)(BNPP)] 3MeCN (3), [Cu2(L)(Fa)] 2DMF (4), [Cu2(L)(Pa)]DMF (5), (H3L=N,N1-bis(3,5-tert-butylsalicylidene-2-hydroxy)-1,3-propandiamine, BNPP=bis(4-nitrophenyl) phosphate, Fa=2-tetrahydrofuroate acid Pac=benzoate) were synthesized and characterized by x-ray single-crystal structure analyses. Variable temperature magnetic susceptibility studies (2-300 K) indicate the existence of ferromagnetic coupling between the (II) ions complexes 1 and 4 antiferromagnetic coupling in complexes 3 and 5.
Three cyanide-liked Fe(III), Mn(III) bimetallic clusters, [(Tp)Te(CN)3]2 [Mn(aacphen)], [1:acphen=N,N1-ethylenebis(2 hydroxyacetophenylideneiminato) dianion, [(Tp(Fe(CN)3], [ Mn(5-bracphen)], [2:5-bracphen=N,N1-ethylenebis(5-bromo 2- hydroxyacetophenylidene iminato) dianion] were prepared, by self-assembling a facial [(Tp)Fe(CN)3], [Tp=hydrolis (pyrazoly) borate] precursor and responsible Mn(III), Schiff bases. Although the geometric parameters relevant to the magnetic Fe-C=N-Mn pathways are analogous to each other their magnetic natures are varied accross the compounds, which support that a degree of orbital overlap is quite sensitive to a subtle structural change in the present system.
Researcher have been synthesized {Cr(CN)4[CNMn(salen)(MeOH)]2} [Mn(salen(CH3OH)(H2O)]} [Mn(salen)(MeOH)]2 [Cr(CN)6]-6CH3OH (1; H2 salen=N,N1-ethylenebis(salicylidene-amine)) ligand and their complexes. All these structural units are linked by hydrogen bonds into a 3D network. The magnetic characterization shows that first complex displays a weak ferromagnetic behavior.
The next work describes synthesis of four trinuclear Cu(II) complexes, [CuL1)3(μ-OH)](NO3)2] (1), [CuL2)3(μ-OH)] (I)2 H2O](2), [CuL3)3 (μ-OH)](I)2 ] (3) and [CuL1)3 (μ-OH)][CuI)2] (4), where HL1 (8-amino-4-methyl-5-azaoct-3-en-2-one), HL2 [7-amino- 4-methyl-5-azaoct-3-en-2-one] and HL3 [7-amino-4-methyl-5-azahept-3-en-2-one] are the three tridentate ligands and characterization.
Magnetic suspectibilities were determined for these complexes at 2 K-300 K. The isotropic hamiltonian, H=- J12, S1, S2-J13, S1S3-J23 S2S3 was used to interpret the magnetic data. The best fit parameteres obtained are J=- 54,98 cm-1, g-2,24 for 1: J=- 56,66 cm-1, g=2,19 for 2; J=-44,39 cm-1, g=2,16 for 3; J=- 89,92 cm-1, g=2,25 for 4. The EPR data at low temperature indicate that the phenomenon of spin frustration occurs for complexes 1-3 [82].
Researcher were synthesized two new azido derivatives of tridentate Schiff base copper (II) complexes. Analyses rveals that [Cu( L1 ) (N3)J (1), containing 1-(salicylideneeimino)-2- (diethylamino) ethane (HL1) as coligand is monomeric in nature while complex [Cu(L2)(N3) Jn(2) containing, 1-(salicylideneimino)-2-(dimetyamino) ethane (HL2) as coligand, has a 1-dimensional infinite chain structure in which copper (II) is square planar in the case of complex 1ç base ligand (HL1) and the fourth site is occupied by an azido group. However, in complex 2 the copper (II) is square planar in the case of complex 1, base ligand (HL1) and thee fourth site is occupied by an azide group.
However, in complex 2, the copper (II) coordination is distorted square pyramidal. The four in plane coordination sites are similar to those in complex 1. The fifth apical coordination is provided by a nitrogen atom of the azido group of a symmetry related moiety with a long Cu-N bond distance, resulting in the polymer of the complex. The variable temperature magnetic susceptibility measurements showed that the magnetic interaction in [Cu(L2)(N3)In (2) is antiferromagnetic (I=-22,5 (± 0.2) sm-1) while as expected [Cu(L1)(N3)I (1) is paramagnetic. The solvent electronic spectra of the complexes show strong absorption bands asssosiated with N3Cu (II) charge transfer transition.
An iron complex [Fe(H5L)] Cl3, of a hexadentate linear Schiff base ligand (H2L) containing O2N4 donor atoms bis (salicylidene) triethylenetetramine, was prepared from FeCl3 and the structure determined with x-ray diffraction. The Fe(II) ion in the reactant is oxidized to form the Fe(III) complex. [C20H24Cl3N4O2]Fe, and the three chloride ions are situated at distance of Fe... Cl (1) 3.976 (5), Fe... Cl (2) 4.479 (3) and Fe... Cl (3) 7.509 (4). A outside the coordination sphere. The Fe(III) ion has an octahedral coordination sphere and as expected the tüo oxygen donor atoms are coordination in a as position. The Fe-Oç Fe-Nimin and Fe- Namine distances are 1.880 (3) A 1.931 (4) A and 2.004 (4) A, responsibility.
The next work describes the synthesis of [Na2L2(μ1.1-N3)2 (N3)2] complexes in the base of reaction beetween Ni(NO3)2 6H2O with L in the presence of excess of sodium azide in methanol at room temperature. Here L is N, N-bis (2-pyridylmethyl amine) (L1) and N-(2-pyridylmethyl-N1, N1-diethylethylenediamine (L2). The x-ray structure of both compounds reveal that the N1N1N coordinating reduced Schiff bases are legated facially. The Ni-Nazido-Ni angle is -1000 and the Ni.....Ni seeepn. Is 3.2 H. The variable temperature magnetic susceptibility measurements of the two complexes show ferromagnetic behavior. Researchers were synthesized three cubane copper (II) clustera, [Cu4 (HL1)4] (1) [Cu4L2 (OH)2] (2) and [Cu4L2 (OMe)2] (3) of two pentadentate Schiff base ligand N1N1-(2-hydroxypropane-1,3-diyl) bis (acetylacetoneimine)(H3L1) and N1N1-(2- hydroxypropane-1,3-diyl) bis (salicylaldimine)(H3L) and magnetic properties studied.
The variable temperature magnetic susceptibility exchange pathway, there is also a weak antiferromagnetic exchange between the copper centers. The theory fitting of the magnetic data gives the following parameteres: I1=38,5 and I2=-18 cm-1 for 1 with a triplet (S=1) ground state and quintet (S=2) lowest excited state: I1=14,7 and I2=-18,4 cm-1 for 2 with a triplet ground state and singlet (S=0) lowest excited state; and I1=33.3 cm-1 and I2=-15 cm-1, 6 cm-1 for 3 with a triplet ground state and quinted lowest excited state, where I1 and I2 are two differet exchane pathways in the cubane ground state and quinted lowest excited state, where I1 and I2 are two different exchange pathways in the cubane (Cu4O4) core. The crystal structures of 26H2O and 32H2O. THF show channels containing the lattice solvent mols.
Conclusion
Schiff bases have been widely explored for industrial applications. However, the biological activity of this class of compounds deserves further investigation. This becomes clear when plant pathogens are considered. Although the research on this subject is incipient, a number of reports disclosing the effects of the Schiff bases on the pathogens of clinical interest have recently been increasing. Schiff base compounds have been shown to be promising leads for the design of more efficient antimicrobial agents. Advances in this field will require analyses of the structure activity relationships of the Schiff bases as well as the mechanism of action of these compounds. The synthesized of Schiff bases have been reported in the above reviewed work gives different approaches to the challenge of preparing these bioactive products and allows the synthesis of many novel chemical derivatives. These derivatives have vast range of biological activities which benefits us.
References
- Schiff H. Communications from the University Laboratory at Pisa: A new series of organic bases. Justus Liebigs Ann Chem. 1864;131(1):118-119.
- Qin W, Long S, Panunzio M, et al. Schiff bases: A short survey on an evergreen chemistry tool. Molecules. 2013;18(10):12264-12289.
[Crossref] [Google Scholar] [PubMed]
- Dhar DN, Taploo CL. Schiff-bases and their applications. J Sci Ind Res. 1982;41(8):501-506.
- Rahimova AR. The role of Schiff Bases in the antibacterial activities and medical applications. J Multidisciplin Eng Sci Technol. 2021;8(10):14626-14640.
- Kumar S, Dhar DN, Saxena PN. Applications of metal complexes of Schiff bases: A review. J Scientific Industrial Res. 2009;68:181-187.
- Rahimova AR. The role of Schiff bases in the antibacterial activities and medical applications.
- Hussain Z, Yousif E, Ahmed A, et al. Synthesis and characterization of Schiff's bases of sulfamethoxazole. Org Med Chem Lett. 2014;4(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Babu K, Amutha P. The new Cu(II) and Ni(II) complexes of schiff bases: Synthesis, characterization and antibacterial studies. Der Pharma Chem. 2014;6:432.
- Badma Priya D, Santha Lakshmi S. Synthesis, spectral and antimicrobial investigation of some ternary Schiff base transition metal complexes. Int J Chemtech Res. 2014;6(1):87.
- Patel A, Bari S, Talele G, et al. Synthesis and antimicrobial activity of some new isatin derivatives.
- Do?ruer DS, Önkol T, Oezkan S, et al. Synthesis and antimicrobial activity of some 3(2H)-pyridazinone and 1(2H)-phthalazinone derivatives. Turk J Chem. 2008;32(4):469-479.
- Vinita G, Sanchita S, Gupta YK. Synthesis and antimicrobial activity of some salicylaldehyde schiff bases of 2-aminopyridine. Res J Chem Sci. 2009;14(8):34-36.
- Shariar SM, Jesmin M, Ali MM. Antibacterial activities of some Schiff bases involving Thiosemicarbazide and Ketones. Int Lett Chem Phy Astron. 2014;7:53-61.
- Gülcan M, Sönmez M, Berber ?. Synthesis, characterization, and antimicrobial activity of a new pyrimidine Schiff base and its Cu(II), Ni(II), Co(II), Pt(II), and Pd(II) complexes. Turk J Chem. 2012;36(1):189-200.
- Hamon F, Djedaini-Pilard F, Barbot F, et al. Azobenzenes-synthesis and carbohydrate applications. Tetrahedron. 2009;49(65):10105-10123.
- Ravoof TB, Crouse KA, Tahir MI, et al. Synthesis, characterization and bioactivity of mixed-ligand Cu(II) complexes containing Schiff bases derived from S-benzyldithiocarbazate and saccharinate ligand and the X-ray crystal structure of the copper-saccharinate complex containing S-benzyl-β-N-(acetylpyrid-2-yl) methylenedithiocarbazate. Polyhedron. 2007;26(6):1159-1165.
- Ali MA, Mirza AH, Bujang FH, et al. Synthesis, characterization and X-ray crystallographic structural study of copper (II) and nickel (II) complexes of the 2-quinoline carboxaldehyde Schiff base of S-methyldithiocarbazate (Hqaldsme). Polyhedron. 2006;25(17):3245-3252.
- Roy S, Mandal TN, Barik AK, et al. Metal complexes of pyrimidine derived ligands-Syntheses, characterization and X-ray crystal structures of Ni(II), Co(III) and Fe(III) complexes of Schiff base ligands derived from S-methyl/S-benzyl dithiocarbazate and 2-S-methylmercapto-6-methylpyrimidine-4-carbaldehyde. Polyhedron. 2007;26(12):2603-2611.
- Akbar Ali M, Huq Mirza A, Ai Fong G. Synthesis, characterization and X-ray crystal structures of the bis-ligand Zinc (II) and Cadmium (II) complexes of the methylpyruvate Schiff base of S-methyldithiocarbazate. Trans Metal Chem. 2004;29:613-619.
- Gou S, You X, Xu Z, et al. Transition metal complexes of the schiff bases derived from S-alkyldithiocarbazate with 2-pyridinecarboxaldehyde N-oxide II. Syntheses, properties and X-ray crystal structure of five-coordinate Copper (II) complexes. Polyhedron. 1991;10(12):1363-1366.
- Ali MA, Mirza AH, Hamid MH, et al. Diphenyltin (IV) complexes of the 2-quinolinecarboxaldehyde Schiff bases of S-methyl-and S-benzyldithiocarbazate (Hqaldsme and Hqaldsbz): X-ray crystal structures of Hqaldsme and two conformers of its Diphenyltin (IV) complex. Polyhedron. 2005;24(3):383-390.
- Ravoof TB, Crouse KA, Tahir MI, et al. Synthesis, characterization and bioactivity of mixed-ligand Cu(II) complexes containing S-methyldithiocarbazate derivatives and saccharinate ligands and the X-ray crystal structure of the copper-saccharinate complex containing S-methyl-β-N-(6-methylpyrid-2-yl) methylenedithiocarbazate. Polyhedron. 2004;23(16):2491-2498.