Original Article
, Volume: 9( 6)A New Sensor for the Determination of p-Aminophenol Based on Screen Printed Electrode
- *Correspondence:
- Mahmoud Khodari , Department of Chemistry, South Valley University, Qena, Egypt, Tel: +2096 5 210 213; E-mail: khodari@svu.edu.eg
Received: November 30, 2016; Accepted: December 09, 2016; Published: December 12, 2016
Citation: Khodari M, Rabee E. A New Sensor for the Determination of p-Aminophenol Based on Screen Printed Electrode. Chemxpress. 2016;9(6):111.
Abstract
A new Voltammetric sensor based on screen printed electrode was established for the determination of p-aminophenol (4-AP), The cyclic voltammograms of 4-aminophenol (4-AP) showed a well-defined peak at -57V and a small peak at -48 mV at low concentration, but this peak shifted to positive values by increasing concentration. The reduction peak was characterized with respect to supporting electrolyte, pH, accumulation potential, scan rate and other different experimental variables. The resulting reduction peak response showed a linear behavior with correlation coefficient of 0.9971. A detection limit of 0.045 μM 4-aminophenol was obtained under the optimum conditions; the method was applied for the determination of the mentioned compound in water and soil samples.
Keywords
Screen printed electrode; Voltammetry; p-aminophenol; Peak response; Determination; 4-Aminophenol
Introduction
There are a number of papers dealing with the determination of 4- aminophenol, p-nitroaniline and 2,4-dinitroaniline in substance, pharmaceutical preparations and biological materials, 4-Aminopehnomay also take part in enzymatic reactions [1]. Used electrochemical and spectral methods to study oxidation reaction of 4-Aminopehnomay by hydrogen peroxide in the presence of horseradish peroxidase as a catalyst [2]. Study the oxidation determination of 4-nitrophenol using fluorescence flow immunoassay (FFIA), which based on the competition between a fluorescein labeled analyte (tracer) derivative and the target compound for a limited amount of anti-4-nitrophenol antibody [3]. Was used the graphene-chitosan composite film modified glassy to determine 4-aminophenol (4-AP). In 0.1 M pH 6.3 phosphate buffer solution, In this work. the used sensors have graphite/Platinum electro deplatinum working electrodes with an area of about 7 mm2. Screen printed electrode were used for the chrono amperemetirc determination of Iodide [4]. Sulphite and nitrite [5-7]. An enhancement selectivity and stability of screen printed electrode for the enzyme electrode was reported. In this work a screen printed electrode was used to determine 4-Aminopehnomay in different media using voltammetric techniques.
Experimental
Preparation of solutions
Stock solutions (0.1 M) of p-aminophenol, were prepared daily, the solutions of lower concentration for experiments were freshly prepared by appropriated dilution from stock solution prior to each run. Other reagents (BDH) were prepared using double distilled water.
Instrumentation
Instrumentation for voltammetric measurements
A computer-aided electrochemistry system used in the voltammetric studies consists of potentiostat model 263 (EG and G PARC) Princeton applied corporation (made in USA) and electroanalytical software model 270/250 version 4.0 (PARC) which control the potentiostat via IEEE 488 GPIB using IBM compatible 386 with VGA monitor and HP Laser Jet 4L printer.
The characteristic of the analyzer potentiostat control of working electrode, which minimize errors from the cell resistance (distorted voltammo gram with decreased peak current and shifted and broadened peaks). This is accomplished with a three-electrode system, the working electrode, the reference electrode and a counter one.
For all the voltammetric measurements the supporting electrolyte was degassed in a 20 ml cell. Then the voltammogram was recorded after applying the required potential for a period of time. The analyte under investigation was added in a definite concentration and the peak response was characterized with respect to pH, deposition period. A stir bar is rotating followed by a rest period and then the scan was initiated in quiescent solutions. All the experiments were carried out at 25°C.
Preparation of the screen-printed electrodes
The used sensors were fabricated at the "Gesells chaftfur Biotechnologies che ForschungmbH (GBF), Institute for Enzyme- technologies "Braun schweig, Germany, using screen printed technology. As reported elsewhere [8]. All measurements were done using planar screen-printed platinum as reference electrode and a platinum wire as a counter electrode. Potentials given with respect to the screen-printed Pt electrode are shifted compared to Ag/AgCl reference electrode by -200 mV, i.e. a potential of -500 mV vs Pt corresponds to -300 mV vs Ag/AgCl.
Instrumentation for pH-metric measurements
The pH-metric measurements were carried out using a Thermo Orion Model 420 A plus digital pH-meter. The electrode was calibrated using standard buffers of pH 4.01, 7.00 and 10.01.
The voltammetric behavior of p-aminophenol was investigated using C/Pt screen printed electrode. The peak was characterized with respect to supporting electrolyte, pH, accumulation potential, scan rate and other different experimental variables.
Results and Discussion
Cyclic voltammetric studies of p-aminophenol
In potassium phosphate (pH=8.00), the cyclic voltammogram of p-aminophenol showed a well-defined peak at -0.57 V and a small peak at -48 mV at low concentration, on increasing concentration of p-aminophenol, the peak shifted to positive values. The repetitive cyclic voltammograms indicated a rapid desorption of the adsorbed form the peak current decreased sharply in the second and third cycles. So, a linear sweep voltammetric technique is preferable in such cases.
Influence of supporting electrolyte
The effect of different supporting electrolytes was examined using a series of electrolytes: sodium acetate, sodium perchlorate, potassium chloride, sodium citrate, sodium nitrate, sodium borate, potassium phosphate, sodium phosphate buffer and Britton-Robinson buffer. The peak height and the peak shape were taken into consideration in choosing the suitable supporting electrolyte. Only five buffers, sodium citrate, sodium borate, sodium phosphate, potassium phosphate and B. R. buffer showed adsorption of p-aminophenol onto C/Pt screen printed electrode, while the other supporting electrolytes didn’t show this behavior, so potassium phosphate was selected for further work because it does not only give the highest peak current but also gave the best peak shape. The effect of the ionic strength of potassium phosphate on the peak response of p-aminophenol was studied over the range 0.01 M to 0.5 M. The best peak response was found at 0.1 M potassium phosphate.
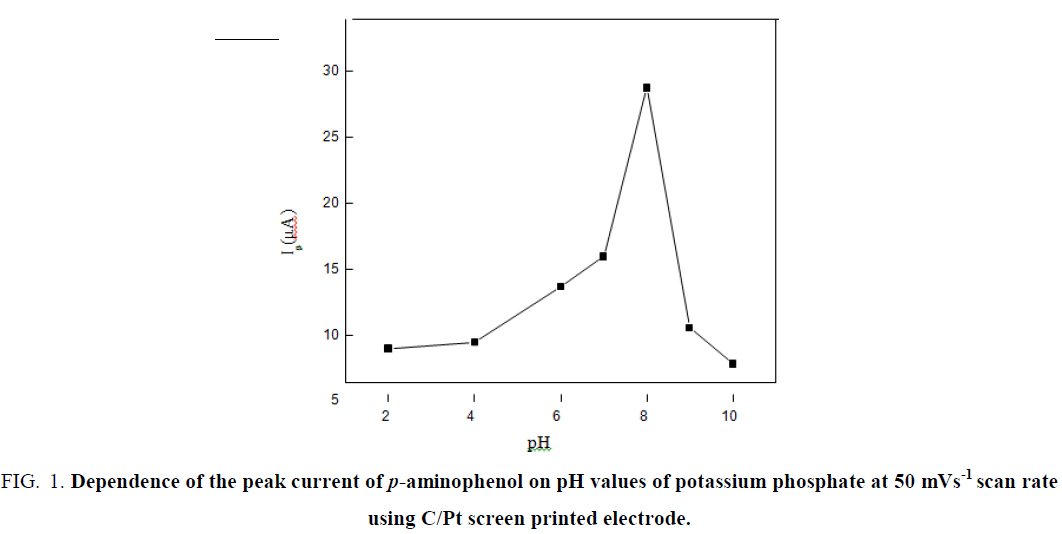
The influence of pH of potassium phosphate solution on the peak current of p-aminophenol was examined (pH 2-12). p-aminophenol showed a small peak current at lower pH values. The peak current increased with increasing the pH of the electrolyte with a maximum value at pH=8.00 (Figure 1), therefore potassium phosphate of (pH=8.00) was selected for further investigations. The effect of pH on the peak potential of the drug was examined; and the results showed that the peak potential shifted to more negative values with raising pH.
Figure 1: Dependence of the peak current of p-aminophenol on pH values of potassium phosphate at 50 mVs-1 scan rate using C/Pt screen printed electrode.
Influence of scan rate and deposition time
By varying the scan rate from 10 to 300 mVs-1, the peak current of 1 × 10-4 mol m-3 p-aminophenol increases with increasing scan rate. It was observed that the peak current increases with increasing scan rate. A 50 mV/s was used to avoid distortion of peak shape in the presence of high concentration of the investigated compound. Figure 1 indicates the effect of scan rate on the peak current of 1 × 10-4 mol m-3 4-aminophenol, where the peak potential shifted to more negative values by increasing of scan rate at scan rate higher than 200 mVs-1, the peak shape was distorted; therefore, a scan rate of 50 mVs-1 is most suitable one.
On plotting logarithm, peak current (log IP) against logarithm scan rate for 1 × 10-4 mol m-3 p-aminophenol, a linear relation was obtained with correlation coefficient of 0.9993 and slope of 0.7137.
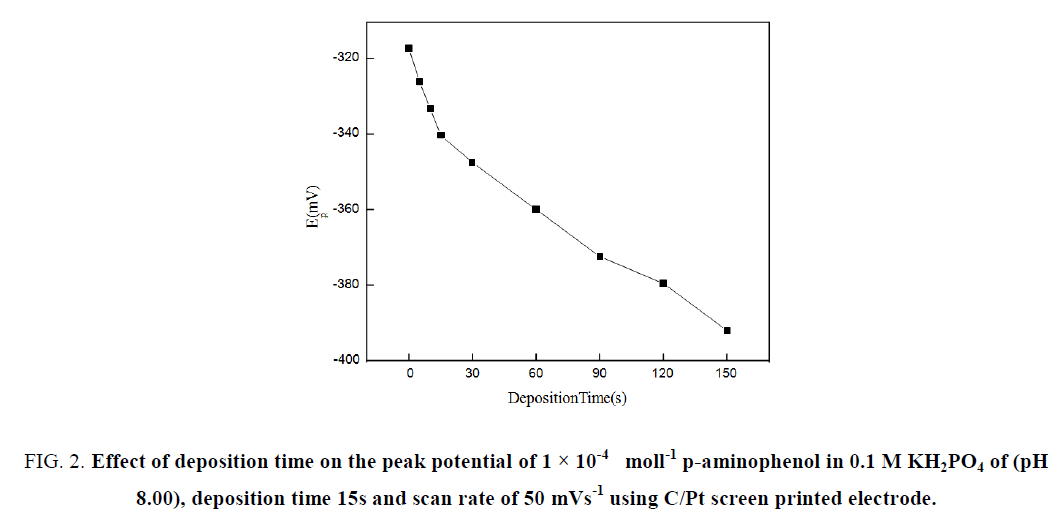
The adsorption behavior of p-aminophenol on C/Pt screen printed electrode has been proved by increasing the peak current with increasing the deposition time over a range 0.0-150 s. Figure 2 shows the influence of deposition time on the peak potential of 1 × 10-4 mol m-3 p-aminophenol.
Figure 2: Effect of deposition time on the peak potential of 1 × 10-4 moll-1 p-aminophenol in 0.1 M KH2PO4 of (pH 8.00), deposition time 15s and scan rate of 50 mVs-1 using C/Pt screen printed electrode.
Reproducibility and sensitivity
The reproducibility of the results was determined by successive measurements of 1 × 10-5 mol m-3 p-aminophenol at C/Pt screen printed electrode, deposition time 15 s and scan rate 50 mVs. The standard deviation was calculated and was found to be 0.47%.
Quantification and detection limits
A Quantification limit of 3 × 10-7 mol m-3 p-aminophenol was obtained in 0.1 M potassium phosphate (pH 8.00), at 15 s deposition time, and 50 mVs-1 scan rate using C/Pt screen printed electrode. A detection limit of 0.045 μM mol m-3 was calculated based on (S/N=3). Table 1 shows the optimum conditions and detection limits for the present and previous work.
| Technique | Working electrode | Supporting electrolyte | Epor E1/2 | Detection Limit |
|---|---|---|---|---|
| Cyclic and Linear Sweep Voltammetry (present work) | Screen printed electrode | KH2PO4 (pH 8.00) | -0.57V | 0.045 μM |
| Voltammetric Technique [3] | Modified GCE | phosphate pH 6.3 | ------ | 0.057 μM |
| Cyclic voltammetry [9] | Modified carbon paste electrode | KCl (pH 6.00) | +325 mV | 3 µM |
Table 1: Comparison between the detection limits for p-amino phenol in the present work.
Influence of interfering species
The influence of some metal ions, urea, L-ascorbic acid, some organic and some amino acids were examined on the peak current of p- aminophenol in potassium phosphate (pH 8.00), 15 s deposition time and scan rate of 50 mVs-1.
Different concentrations of Cu (II) ranged from 5 × 10-6 to 5 × 10-5 mol m-3 were added to 5 × 10-5 mol m-3 4-aminophenol. A concentration of 2 × 10-5 and, 5 × 10-5 mol m-3 Cu (II) decreased the peak current by about 1.5% and 2.9% respectively. Concentration of 2 × 10-5 and 5 × 10-5 mol m-3 Pb(II) reduced the peak current response of 5 × 10-5 mol m-3 p-aminophenol by about 3.3% and 4.1% respectively. The peak current of 5 × 10-5 mol m-3 p-aminophenol was reduced by about 1.8% and 2.7% on addition of 2 × 10-5 and 5 × 10-5 mol m-3 Cd(II), where other concentrations such as 5 × 10-6 and 1 × 10-5 mol m-3 have no effect on the peak current response of the studied compound.
Addition of 2 × 10-5 and 5 × 10-5 mol l-1 L-alanine reduced the peak current of 5 × 10-5 mol l-1 p-aminophenol by about 2.8% and 3.2% respectively, where the addition of 5 × 10-5 mol l-1 glycine and L-leucine depressed the peak current of p-aminophenol by about 3.4% and 2.9% respectively.
Addition of 2 × 10-5 and 5 × 10-5 mol l-1 of L-ascorbic acid increase the peak current of 5 × 10-5 mol l-1 of p-aminophenol by about 4.6% and 5.1% respectively, while the concentrations less than 2 × 10-5 mol l-1 have no effect on the peak current response.
Analytical applications
Under the optimum conditions, p-aminophenol has been determined in water and soil samples to determine p-aminophenol, in water samples, linear sweep voltammograms were recorded before and after adding different concentrations of p-aminophenol to a voltammetric cell containing 10 ml of water sample and 10 ml potassium phosphate solution. A 15 s deposition time and scan rate of 50 mVs-1 were applied. The obtained results are shown in Table 2. The relative standard deviation shown in the table has been calculated on five replicate determinations.
| Samples No. | DL(µgl-1) | RSD% | Recovery (R%) |
| 1 | 3.64 | 0.25 | 98.6 ± 1.8 |
| 2 | 5.38 | 0.36 | 97.3 ± 2.3 |
| 3 | 7.52 | 0.28 | 99 ± 3.1 |
| 4 | 3.34 | 0.31 | 101.2 ± 3.8 |
| Industrial wastewater | 13.45 | 0.27 | 98.4± 2.7 |
Table 2: Determination of 4-aminophenolin water samples.
p-aminophenol was determined in soil samples by recording voltammograms in the absence and presence of p-aminophenol to a voltammetric cell containing 10 ml of extracted soil sample and 10 ml potassium phosphate solution, the results are reported in Table 3.
| Samples No. | DL (µgl-1) | RSD% | Recovery (R%) |
| 1 | 15.82 | 0.35 | 99.2 ± 3.2 |
| 2 | 31.59 | 0.32 | 100 ± 1.5 |
| 3 | 10.47 | 0.39 | 98.5 ± 2.4 |
| 4 | 23.67 | 0.25 | 99.8 ± 1.8 |
| 5 | 17.52 | 0.16 | 101.3 ± 1.4 |
Table 3: Determination of 4-aminophenolin soil samples.
Conclusion
The screen printed electrode was prepared carefully and used for determination of 4-Aminophenol using differential pulse voltammetry technique. The mechanistic studies of electrochemical process of 4-Aminophenol was investigated using cyclic voltammetry technique, the differential pulse voltammetric technique using C/Pt screen printed electrode enhanced the limit of detection based on (S/N=3), and a value of 0.045 μM was obtained. Repeatability of 10 runs of 1 × 10-5 mol m-3 4-aminophenol gave a standard deviation of 0.47%. The electrode was used to determine the compound under investigation in water and soil samples.
Acknowledgment
The screen printed electrodes are gift from Prof. U. Bilitewski Gesellschaft fur Biotechnology sche For schungmbH (GBF), Institute fur Enzyme- technologies" Braunschweig, Germany, and the authors acknowledge Prof. U. Bilitewski.
References
- Zhang S, Jiao K, Li HJ, et al. Chem J Chin Univ. 2000; 21:710.
- Nistor C, Oubina A, Marco MP, et al. Competitive flow immunoassay with fluorescence detection for determination of 4-nitrophenol. Anal. Chim. Acta. 2001;426:185-95.
- Yina H, Maa Q, Zhoua Y, et al. Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene–chitosan composite film modified glassy carbon electrode. Electrochimica Acta. 2010;55:7102-8.
- Khodari M, Bilitewski U, Bassry A. Screen-printed electrodes for amperometric determination of Iodide. Electro analysis.2014;27:281-84.
- Khodari M. Selective electrochemical determination of desipramine using a lipid modified carbon paste electrode. Int J Sci Res. 2014;12(3):1536-39.
- Khodari M, Basry A, Mersal G. Electrochemical sensor based on carbon printed electrode. Int J Sci Res. 2014;12(3):1406-9.
- Mersal G, Khodari M, Bilitewski U. Optimisation of the composition of a screen-printed acrylate polymer enzyme layer with respect to an improved selectivity and stability of enzyme electrodes. Biosens Bioelectron. 2004;20:305-14.
- Bilitewski U, Ruger P, Weise W. GBF Monograph. Biosensors: Fundamentals, technologies and Application. Weinheim: VCH, Vol 17; 1992, p. 199.
- Cardoso WS, Kubota LT, Gushikem Y. Electrocatalytic oxidation of phenolic compounds using an electrode modified with Ni(II) porphyrin adsorbed on SiO2/Nb2O5-phosphate synthesized by the sol-gel method. J Electroanal Chem. 2007;602:29-36.