Research
, Volume: 19( 1) DOI: 10.37532/0974-7419.2019.19(1).1443R Potentiometric Membrane for In-Line Environmental Monitoring of Cu2+ in Swimming Water
- *Correspondence:
- Hebatallah M Essam Analytical Chemistry Department, Faculty of Pharmacy, Cairo University, Kasr-El Aini Street, 11562 Cairo, Egypt, E-Mail: hebatallah.essameldin@pharma.cu.edu.eg
Received: January 07, 2018; Accepted: January 14, 2018;; Published: January 21, 2018
Citation: Hebatallah M Essam, Mohamed K Abd El-Rahman. 3R Potentiometric Membrane for In-Line Environmental Monitoring of Cu2+ in Swimming Water. Anal Chem Ind J. 2019;19(1):144.
Abstract
The idea of green chemistry has become the most interesting area for research, not only for clean environmental resources but also for whatever we do and however we do. Then, the fundamental goal of green chemistry research is to reduce pouring stream of chemicals for hazards purification. Consequently, the concentration of Cu2+ ions in swimming pools that use Copper ionizers as a disinfectant requires periodic monitoring to be maintained within safe limits. So, a 3R (Reduce cost, waste and solvents, Real time analysis method, Reusable) copper (II) selective electrode with the firstly introduced 2-amino-4-chlorophenol (ACP) was proposed. Confirmatory spectrophotometric study was used to investigate the complex stability and formation ratio between Cu2+ and ACP. Selectivity investigations showed exceptional selectivity for Cu2+ ions over interfering cations in comparison to the usually used sulphur-containing ionophores. The proposed sensor showed successful applications to the in-line determination of copper ions in swimming pools water operated by copper ionization system as a chlorine alternative for disinfection. Statistical studies showed that the suggested technique strategy with such benign design is a superior choice rather than the commonly used off-line technique as atomic absorption that maintains pure planet without the introduction of further hazards into the biosphere.
Keywords
Green chemistry; Potentiometric sensors; Copper determination; Swimming pools water; 2-amino-4-chlorophenol
Introduction
Chlorine-based pump is the most commonly used procedure for primary disinfection of swimming pools. However, there are many concerns of chlorine being toxic with a suffocating odor and burning-eye cause, irritation of skin, and damage of hair. It is also absorbed through the skin and having direct links to cancer [1,2]. Recent studies show that swimming in chlorinated pools during childhood is strongly associated with lower levels of serum inhibin B and total testosterone due to the absorption of reprotoxic chlorination by-products across the highly permeable scrotum [3]. Moreover, it was proven that chlorinated pool exposure exerts an adjuvant effect on atopy that seems to contribute significantly to the burden of asthma and respiratory allergies among adolescents [4].
Now, more than ever, a chlorine free pool is a highly desired strategy. Copper ionizers that use a low voltage current across copper bars to free copper ions into the flow of pool water to kill pathogens have become more popular and the best chlorine alternative. It is viewed as the twenty-one century pool disinfection. The process of ionization developed by NASA is the only method that produces pure, odorless, crystal-clear healthy pool water. Also it is the least expensive and easiest of all disinfecting methods to maintain and monitor. Research in this area to determine the effectiveness of ionization to kill bacteria showed that ionization is faster acting and longer lasting than chlorine [5].
The copper ionizers are designed to produce ion concentrations in the range of 0.4-0.8 ppm for copper (6.2 × 10-6 to 1.2 × 10-5 molL-1). Due to its vital role and upon the discovery that certain diseases were transmitted by water as a consequence of decreased concentrations of copper, and to avoid the buildup of excessive level of copper which correlates with some unhealthy effects as Wilson's disease [6] and green hair [7], where, currently, chelating agents are used to reduce the copper content. To this end, the concentrations of copper ions require periodic testing and close monitoring to ensure the recommended levels are maintained. The currently used commercial methods for determination of copper content depend on dipping a strip in the water and compare the color change to a number/color scale or reagent test kits that depends on visual color matching, which considered being more accurate on a qualitative level.
The motivation to develop new sensor is not only the achievement of better potentiometric performance parameters but also to solve problems in actual applications in agreement with the famous 12 principles of green chemistry. Over the last two decades, ISEs have acquired increasing prominence due to several advantages such as portability, low energy consumption, limited sample pretreatment, rapidity, miniaturization capability, being non-destructive and adaptability to small sample volumes and on-site monitoring. Moreover, their ability to integrate with microfabrication processes allows the mass fabrication of cost-effective sensors [8]. Such desirable characteristics have made ISEs the center of considerable attention across many disciplines of science, and they have potential applications of clinical, environmental, and forensic analyses [9,10].
In view of such advantages, a number of Cu2+ selective sensors have been developed using solid membranes of insoluble salts of copper [11], copper chelates [12], macrocyclic polyethers [13], noncyclic neutral ionophores containing dithiocarbamate groups [14] and calix [4] arenes [15]. In addition to these solid membrane sensors, some liquid membrane sensors using Cu (II) complexes [16] have also been reported. In spite of availability of number of Cu2+ sensors with remarkable selectivity, their use in actual applications for Cu2+ estimation has been limited, From these sensors, only the electrodes [17,18] were recommended for determination of Cu2+ in vegetable foliar and drinking water.
The present work describes the applications and advantages of 2-amino-4-chloro-phenol (sulfur-free compound) as a neutral ionophore for the development of 3R (Reduce cost, waste and solvents, Real time analysis, Reusable) sensor for the determination of Cu2+ with spectrophotometric study of the complex stability and ratio of formation. This sensor was used for the determination of Cu2+ in different swimming pool water operated by copper ionizers. In addition to the 3R advantages, Using sulfur free ionophore, presents eco-friendly electrode with a smart management of ACP, which is a chemical by-product.
Materials and Methods
Chemicals and reagents
All chemicals and reagents used were of analytical reagent grade. 2-amino-4-chlorophenol was obtained from Sigma (St. Louis, USA). Water was bi-distilled. Polyvinyl Chloride (PVC), was obtained from Fluka (Steinheim, Germany). 2-Nitrophenyl octyl ether (NPOE) and sodium tetraphenyl borate (NaTPB) were purchased from Aldrich (Steinheim, Germany). Tetrahydrofuran (THF) was obtained from BDH (Poole, England). Potassium chloride and Copper (II) sulphate pentahydrate were obtained from Prolabo (Pennsylvania, USA).
Fabrication and construction of membrane sensors
The PVC membrane was prepared in 5-cm Petri dishes by mixing 199.4 mg (33.23%) PVC powder, 400 mg (66.6%) NPOE, 0.17 % (5 mM/kg) sodium tetraphenylborate (NaTPB), and 0.21% (15 mM/kg) ACP. The membrane components (totaling 600 mg) were dissolved in minimum amount of THF, and the Petri dish was covered with filter paper and left to stand overnight at room temperature to allow solvent evaporation. Master membrane 0.1 mm in thickness was obtained. From master membrane, a disk (about 8 mm in diameter) was cut using a cork borer and pasted using THF to an interchangeable PVC tip that was clipped into the end of an electrode glass body. The electrode was then filled with an internal solution of equal volumes of 10-4 molL-1 Cu2+ and 10-4 molL-1 KCl. Ag/AgCl wire (1 mm diameter) was used as an internal reference electrode. The sensor was conditioned by soaking in 10-4 molL-1 aqueous Cu2+ solution for 24 h, and it was stored in the same solution when not in use.
Potentiometric and spectrophotometric measurements
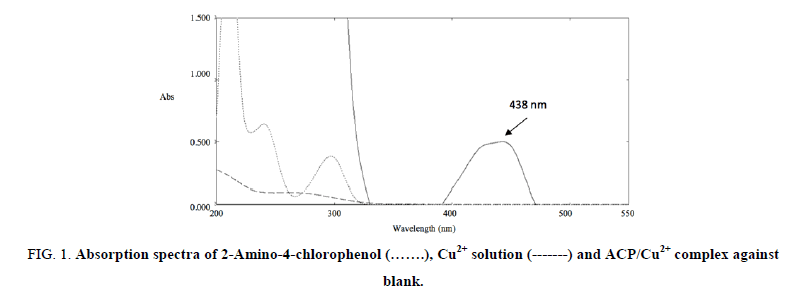
Potentiometric measurements were carried out using an Ag/AgCl double-junction type external reference electrode (Thermo Scientific Orion 900200, MA, USA; 3.0 M KCl saturated with AgCl as inner filling solution and 10% KNO3 as bridge electrolyte) and Jenway digital ion analyser model 3330 (Essex, UK). A Jenway pH glass electrode (Essex, UK) was used for pH adjustments. Calibrations of Cu2+ were determined by a stepwise dilution of Cu2+ solution in deionized water (pH of 5.5, acidified with 0.1M HNO3) and continuous EMF measurements. Selectivity coefficients were determined with the Modified Separate Solutions Method (MSSM) [19]. The electrode was first conditioned in 1 mM NaNO3 to characterize the response performance of the membrane void of primary ions, subsequently; calibration curves for other cations were obtained analogously by successive dilutions. Nernstian responses to the cations were confirmed for all single salt solutions in the concentration range where selectivities were tested and hence unbiased selectivity coefficients [20]. UV spectrophotometric measurements were carried out with SHIMADZU dual beam UV-visible spectrophotometer (Kyoto, Japan), model UV-1650. The study was performed by recording UV/Vis spectra from 200 nm to 550 nm, then measuring the absorbance at 438 nm that corresponds to the ACP/ Cu2+ complex.
Analytical application in swimming pool samples
This section demonstrates the analytical power of the suggested sensor for the detection of Cu2+ in swimming pool water operated by copper ionizers. Cu2+ was measured by applying the described sensor to 2 swimming pool water operated by copper ionizers in Cairo and the north coast of Egypt. Samples are collected from the pools before and after running of the copper ionizers. The water samples were acidified with 0.1M HNO3 to reach a stable pH of 5.5 ± 0.2. Potentiometric measurements were taken with the proposed sensor. Calibration curves were recorded by successive dilutions in the range 10-2 to 10-7 molL-1 CuSO4 using the water samples collected before running of the ionizers as background. Three replicate measurements were performed, and the Cu2+ calibration curves in the swimming pool water matrix overlapped well with the ones measured in deionized water (pH of 5.5, acidified with 0.1M HNO3). The switch from deionized water to swimming pool water resulted in changes in slopes and intercepts (Eo) of the calibration curves of only 0.01% and 1.16%. These differences are not statistically significant and show that this sensor can be used successfully for direct measurements of Cu2+ in swimming pool water samples after running of the ionizers.
Subsequently, the ISE was immersed into 50 mL of the swimming pool samples after the running of the ionizers and the emf was recorded. The validity of the results was demonstrated by comparing the potentiometric results with the measurements of the same samples using atomic absorption instrument (Perkin Elmer Sciex, ELAN 6100 DRC Plus) which was calibrated using prepared standard solutions.
Results and Discussion
Since the use of thiocrown ether, dithizone and the open-chain thioether as the first electrically neutral ionophores for Cu2+ was reported in 1988 [21], many new ionophores have been applied for the construction of Cu2+ ISEs. In most cases, sulfur was the key atom of the coordinating group (s). For example, ionophores with thioether [22] thioate [23] and thiocarbamate groups were reported. The Cu2+ binds to these functional groups with high selectivity. This can be rationalized on the basis of the Hard-Soft Acid Base (HSAB) theory, which predicts that sulfur as a soft Lewis base has a high affinity and is a good coordinating site for Cu2+ as a soft Lewis acid [24].
In many cases, it appears sufficient to design an ionophore that interacts much more strongly with the analyte ion than with the interfering ions of major concern, abandoning the ultimate ionophore selectivity. The present work evaluates the possibility of using the first introduced ligand for metal ion (ACP) as a sulfur free ionophore in the preparation of Cu2+ selective electrode using PVC as a polymeric matrix which offers much more advantageous technique than the conventional atomic absorption one. Also, the proposed membrane has less environmental hazards than sulfur containing membranes in order to reach clean and safe environment through eco-friendly approaches.
Preliminary studies of ACP/Cu2+ complex
As a starting point and before moving to ISE performance, binding assays between Cu2+ and ACP were carried out to confirm that this was a suitable ionophore for Cu2+ and which would allow ISEs with good selectivity features to be prepared. Job’s method of continuous variations was applied in attempts to calculate the empirical formula of the complex and its stability constant, FIG. 1.
FIG 1: Absorption spectra of 2-Amino-4-chlorophenol (…….), Cu2+ solution (-------) and ACP/Cu2+ complex against blank.
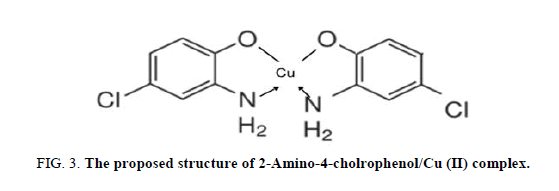
By plotting job’s method curve, it was found that ACP interacts with Cu2+ in the ratio 2:1, FIG. 2.
FIG 2: Determination of the stoichiometry of the reaction between 2-Amino-4-chlorophenol and Cu2+ by the continuous variation method using 4 × 10-4 M solutions.
The value of the stability constant was found to be 8.6 × 1010 Lmol-1 according to the following formula [25].
(ß) = A/Aexp.Cx/(CM-A/Aexp.Cx) (CL- n A/Aexp.Cx)n
Where M=metal, L=ligand, x=mole fraction of the ligand, n=x/1-x, A/Aexp.=ratio of the observed absorbance to the absorbance indicated by the tangent for the same wavelength, CM and CL=molar concentrations of the metal and ligand, respectively. It follows that: Cx=CL/n=CM.
The high binding affinity of the Cu2+/ACP complex can be explained in analogy to most of the better-known organic ligands containing both an acidic and a basic functional group (8-hydroxy quinoline, EDTA) which form chelate compounds with cations. Where the metal interacts with both of these groups, itself becomes one member of a heterocyclic ring. In ACP, the hydroxyl group is located with respect to the nitrogen atom in position that permits the closure of such rings, forms a strong complex with copper ions; the metal ion replaces the acidic hydrogen of the hydroxyl group. At the same time, the unshared pair of electrons on the nitrogen is donated to the Cu2+, thereby forming a five-membered ring, which is very stable in view of the strain theory of organic chemistry, FIG. 3.
Performance characteristics of Cu2+ sensor
The effect of the ratio of ionophore to ionic sites on the potentiometric selectivities and the detection limit was investigated. As well documented, the optimum ratio in view of selectivity and LOD is controlled by the complex stoichiometry and, therefore, the coordination number, of the primary and interfering ions [26]. For Cu2+ the most common coordination number in complexes with inorganic and organic ligands is four [24]. This is in well agreement with our job’s plot for studying the stoichiometry of the ACP/Cu2+ complex.
It is interesting to note that the ionophore and ionic sites are buffering the primary ion activity in the sensing membrane, stronger binding of the primary ion to the ionophore resulting in a lower primary ion activity in the membrane. Under circumstances where the detection limit is not determined by interfering ions but by the release of primary ions from the membrane into the sample, a lower primary ion activity in the membrane is also expected to result in a lower detection limit 42. Therefore, if the concentration of ionic sites is kept constant while the ionophore concentration in the sensing membrane is increased and, thereby the concentration of free analyte ions in the sensing membrane decreases, the detection limit is expected to improve. Experimentally, the molar ratio of the Cu2+ ionophore ACP and the ionic sites in the sensing membrane was varied, while the weight percentages of PVC and NPOE were kept constant. Two molar ratios of ionophore and ionic sites, that is, 3:1 and 2:1, were tested. With the ratio of ionophore to ionic site ratio of 3:1, the detection limit was 1.6 × 10-7 M, which was approximately one order of magnitude lower than for electrodes with the ionophore:ionic site ratio of 2:1 (1.2 × 10-6 M). This seems to be consistent with the fact that the activity of Cu2+ in the membranes with the 3:1 ionophore to site ratio is calculated from the studied 1:2 binding constants to be lower than in the membranes with the 2:1 ratio.
The electrochemical performance characteristics of the proposed sensor were systematically evaluated according to IUPAC standards [27]. TABLE 1 shows the results obtained over a period of two months for two different assemblies of the sensor.
| Parameter | Proposed sensor |
|---|---|
| Slope (mV/decade)a | 28.6 |
| Intercept (mV) | 190.2 |
| LOD (molL-1)b | 1.6 × 10-6 |
| Response Time (sec.) | 8 |
| Working pH Range | 4-7 |
| Concentration Range (mol L-1) | 3.6 × 10-6 × 10-1 |
| Stability (days) | 40 |
| Correlation Coefficient | 0.9998 |
| Ruggednessc | 99.79 |
a. Average of five determinations
b. Limit of Detection (measured by interception of the extrapolated arms of FIG. 3) c. Average recovery percent of determining 10-3 and 10-3 molL-1 Cu2+ for the studied electrodes using Jenway 3510 digital ion analyser instead of the 3310 model
TABLE 1. Electrochemical response characteristics of the investigated Cu2+ sensor.
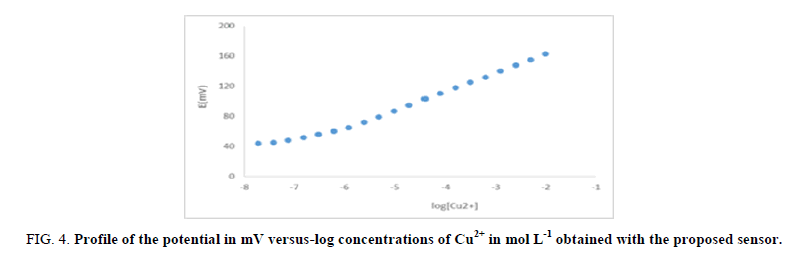
The slope of the calibration plot is 28.6 mV/concentration decade as shown in FIG. 4. Deviation from the ideal Nernstian slope (30 mV) is due to that the electrode responds to the activity of the metal ion rather than its concentration. The sensor displayed constant potential readings for day-to-day measurements, and the calibration slope did not change by more than ± 2 mV/decade over a period of 40 days. The detection limit of the sensor was estimated according to the IUPAC definition 43. TABLE 1 shows that the proposed sensor can detect Cu2+ in very dilute solutions down to 1.6 × 10-7 molL-1 (0.010 ppm), which is 40 times lower than the minimum concentration of Cu2+ in swimming pools water samples operated by Cu2+ ionizers. In this regard, it should be noted that a reliable analytical technique should show detection limits that are at least 10 times lower than the measured concentration.
FIG 4: Profile of the potential in mV versus-log concentrations of Cu2+ in mol L-1 obtained with the proposed sensor.
Dynamic response time
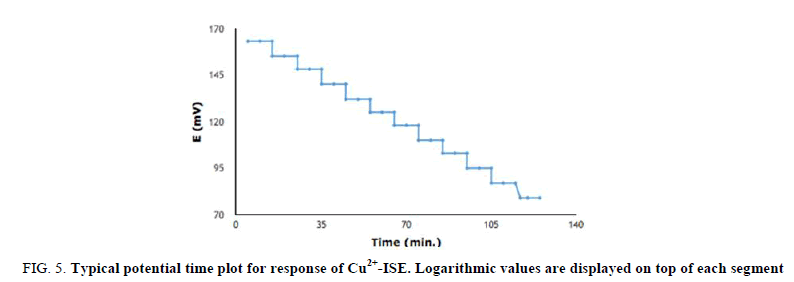
Dynamic response time is an important factor for analytical applications of ISEs, where the most important goal is to capture large amount of data in a very short time. In this study, the time trace of the calibration curve of Cu2+ ISE is represented in FIG. 5. The response times in samples with concentration higher than 10-5 molL-1 were of the order of seconds (less than 5 seconds), whereas in more diluted samples the time needed to reach a stable potential was considerably longer around 15 seconds.
FIG 5: Typical potential time plot for response of Cu2+-ISE. Logarithmic values are displayed on top of each segment
Effect of pH
The effect of pH of the Cu2+ test solutions (1.0 × 10-3 and 1.0 × 10-4 molL-1) on the sensor potential was investigated by following the potential variation over the pH range 2.0-10.0. The potential remains constant over the 4.0-7.0 pH range, beyond which the potential changes considerably. The observed drift at higher pH values could be due to the formation in solution of some hydroxo complexes of Cu2+ or even to Cu(OH)2 precipitate [28]. The observed change in potential at low pH values could be due to protonation of ionophore, which results in an increased potential of the system by increasing concentration of proton in solution, Since the potential remains constant over pH 4.0-7.0, this can be taken as the working pH range for the proposed electrode systems; in particular, pH 5.5 was used for all the experiments.
Sensor selectivity
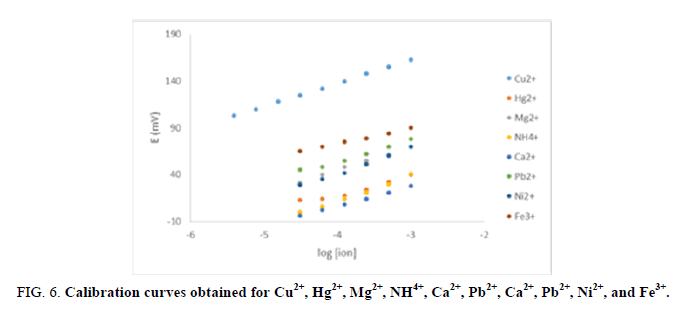
The selectivity of membrane sensor is one of the most important performance parameter th at determines the utility of the sensor for the proposed analytical applications. In order to evaluate this issue, separate calibration curves for a number of ions on membranes conditioned in 1 mM sodium nitrate were performed and the most preferred Cu2+ ion was measured at the end of the experiment, FIG. 6. As established [29], such a protocol allows one to obtain near-Nernstian response slopes, and hence unbiased thermodynamic selectivity coefficients. FIG. 6 shows that obvious near-Nernstian slopes for mono-, di-, and trivalent cations and thus unbiased selectivity coefficients were obtained. This can be attributed to the fact that these ions are easily ion-exchanged into the membrane. It is worth noting that all response times in the Nernstian region were shorter than 20s, and the logarithmic values are averages for three electrodes. The calculated selectivity coefficients suggest a somewhat greater Cu2+ selectivity compared to literature values based on the previously reported sulfur-containing ionophores [21-23], TABLE 2. This can be attributed to couple of reasons; the preferential interaction between the Cu2+ and the acidic hydroxyl group and basic amino group present in the ACP structure, and the absence of the sulfur atom in the ionophore structure which is considered the best coordinating atom for most potential interferents (alkali and alkaline earth metal ions).
FIG 6: Calibration curves obtained for Cu2+, Hg2+, Mg2+, NH4+, Ca2+, Pb2+, Ca2+, Pb2+, Ni2+, and Fe3+.
| Interferent b | Selectivity coefficienta |
|---|---|
| Ca2+ | -4.6 |
| Cr3+ | -2.1 |
| Ni2+ | -3.2 |
| Mg2+ | -3.2 |
| K+ | -3.4 |
| Pb2+ | -1.7 |
| Fe3+ | -2.5 |
| Cd2+ | -2.9 |
| Hg2+ | -4.2 |
| NH4+ | -4.2 |
| Zn2+ | -2.9 |
| Urea | -4.1 |
| Cyanuric acid | -3.7 |
a. Each value is the average of three determinations
b. All interferents are in the form of 1 × 10-3 mol L-1 solution
TABLE 2. Potentiometric selectivity coefficients (Kpot.I) of the proposed sensor using the separate solutions method (SSM) 31.
In parallel investigations to assess the effect of other compounds that are usually found in swimming pools such as urea and cyanuric acid on the response of the proposed sensor, it was found that those components did not show any interference most likely due to the lack of electoactive group. These results encourage the application of the proposed sensor for the analysis of swimming pool water samples without prior treatment or extraction.
Analytical application
In order to avoid interference from H+, the pH was adjusted to 5.5 with 0.1 molL-1 HNO3. At this pH, according to speciation calculations [30-32], 95.5% of Cu2+ occurs in its free form. The calibration curves taken in the pool water samples collected before running of the ionizers as background indicate a detection limit of 7.9 × 10-7 molL-1 (0.05 ppm), which permits direct analysis of Cu2+ in two different swimming pool water samples, TABLE 3. It was found that the proposed sensor is reliable and gives stable results with very good accuracy and high percentage recovery without preliminary extraction procedures. The sample concentration of Cu2+ was then validated with atomic absorption measurements that showed no significant difference between the results. This demonstrates the utility of this ISE for Cu2+ monitoring in swimming pools water samples operated by Cu2+ ionizers.
| Concentration of Cu2+ ± S.D.b | ||
|---|---|---|
| Sample (pool no.)a | ISE | Atomic absorptionc |
| Pool 1, (ppm) | 0.912 ± 0.081 | 0.90 ± 0.054 |
| (µM) | 14.4 ± 0.86 | 14.2 ± 0.66 |
| Pool 2, (ppm) | 1.021 ± 0.076 | 1.19 ± 0.064 |
| (µM) | 15.9 ± 0.53 | 18.7 ± 0.41 |
a. Pool 1: Cairo swimming pool water, pool 2: water taken from pool present in the north coast of Egypt
b. Average of five determinations
c. An Avanta atomic absorbtion spectrophotometer with air acetylene flame was used at 217.0 nm to analyze real samples
TABLE 3. Determination of Cu2+ in swimming pools water samples by the proposed electrode and atomic absorption technique.
Conclusion
From practical view, Ion-selective electrodes are an attractive alternative for routine monitoring of swimming pools water operated by copper ionization system since they are cost-effective, miniaturizable, portable ideally consume nearly no analyte during the measurement, and may provide additional information on the speciation of analytes. Comparison with atomic absorption method shows that direct potentiometric Cu2+ determinations of various swimming pools water samples are conveniently possible. The firstly introduced sulfur-free ionophore (ACP) has shown it to be of superior selectivity for Cu2+ ions over different inorganic ions. The proposed 3R sensor offers advantages of fast response and high selectivity due to the formation of stable complex with Cu2+ ions and being sulfur-free offers a benign design. Therefore, it can be put to analytical use by direct potentiometry, and for routine monitoring of Cu2+ in swimming pools water operated by copper ionization system. Moreover, the overall advantages of ISEs is minimum sample preparation, simple, economic and allows in field measurements.
Synopsis
3R electrode allows real-time measurements to control hazardous analyte with minimum derivatives, which is in reasonable agreement with green chemistry (FIG. 7).
References
- Bakker E. Determination of unbiased selectivity coefficients of neutral carrier-based cation-selective electrodes. Anal Chem. 1997;69:1061.
- Bakker E, Diamond D, Lewenstam A, et al. Ion sensors: Current limits and new trends. Anal Chim Acta. 1999;393:11-8.
- Bakker E, Pretsch E. Potentiometry at trace levels. Trends Anal Chem (TrAC). 2001;20:11-9.
- Bernard A, Nickmilder M, Voisin C, et al. Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics. 2009;24:1110-8.
- Sendra BJM, Lopez AE, Campana GAM. et al. Data analysis in the determination of stoichiometries and stability constants of complexes. Anal Sci. 2003;19:1431.
- Brzozka Z. Copper membrane-electrode based on a macrocyclic thioether. Chem Anal (Warsaw). 1990;35:415.
- Brzozka Z. Transition metal ion-selective membrane electrodes based on complexing compounds with heteroatoms. Analyst. 1998;113:1803.
- Bühlmann P, Chen LD. Ion-selective electrodes with ionophore-doped sensing membranes. Supramolecular Chemistry: From Molecules to Nanomaterials. 2012;5:2539-80.
- Buzuk M, Brinić S, Generalić E, et al. Copper (II) ion selective PVC membrane electrode based on S,S'-bis(2-aminophenyl)ethanebis(thioate), Croat. Chem Acta. 2009;82:801.
- Cobben PLHM, Egberink RJM, Bomer JB, et al. transduction of selective recognition of heavy metal ions by chemically modified field effect transistors (CHEMFETs). J Am Chem Soc. 1992;114:10573-82.
- Florentin, A, Hautemanière A, Hartemann P. Health effects of disinfection by-products in chlorinated swimming pools. Int J Hyg Environ Health. 2011;214:461-9.
- Kamata S, Yamasaki Y, Higo M, et al. Copper (II)-selective electrodes based on macrocyclic polythiaethers. Analyst. 1988;113:45.
- Lewenstam A, Zurawska MM, Hulanicki A. Application of ion-selective electrodes in clinical analysis. Electroanalysis. 1991;3:727-34.
- Nickmilder M, Bernard A. Associations between testicular hormones at adolescence and attendance at chlorinated swimming pools during childhood. Int J Androl. 2011;34:446-58.
- Park SJ, Shon OJ, Rim JA, et al. Calixazacrown ethers for copper (II) ion-selective electrode. Talanta. 2001;55:297.
- Ren K. A liquid-state copper (II) ion-selective electrode containing a complex of Cu (II) with salicylaniline. Talanta. 1989;36:767.
- Sadeghi S, Eslahi M, Naseri MA, et al. Copper ion selective membrane electrodes based on some Schiff base derivatives. Electroanalysis. 2003;15:1327-33.
- Shamsipur M, Javanbakht M, Mousavi MF, et al. Copper (II)-selective membrane electrodes based on some recently synthesized mixed aza-thioether crowns containing a 1,10-phenanthroline sub-unit. Talanta. 2001;55:1047.
- Shvedene NV, Sheina NM, Silasie GV. Liquid-and solid-state coppersensitive electrodes with a membrane based on N-aryl-substituted hydroxamic acid chelates. J Anal Chem USSR. 1991;46:339.
- Pleniceanu M, Preda M, Muresan M, et al. New liquid-membrane electrodes used for the determination of copper and nickel. Anal Lett. 1996;29:1485.
- Sillen LG, Martell AE. Stability constants of metal-ion complexes, Special Publication, The Chemical Society, London, No. 17, 1964.
- Sillen LG, Martell AE. Stability constants of metal-ion complexes, Supplement No. 1, Special Publication, The Chemical Society, London, No. 25, 1971.
- Singh LP, Bhatnagar JM. Copper (II) selective electrochemical sensor based on Schiff Base complexes. Talanta. 2004;64:313.
- Stumm W, Morgan JJ. Aquatic Chemistry, 3rd ed., Wiley, New York, 1996.
- Sun CQ, Sun YP, Zhang X, et al. Selective potentiometric determination of copper (II) ions by use of a molecular deposition film electrode based on water-soluble copper phthalocyanine. Anal Chim Acta. 1995;312:207.
- Szigetti Z, Bitter I, Tóth K, et al. A novel polymeric membrane electrode for the potentiometric analysis of Cu2+ in drinking water, Anal Chim Acta. 2005;532:129.
- Umezawa Y, Buhlmann P, Umezawa K, et al. IUPAC, Analytical Chemistry Division, Commission on Analytical Nomenclature, Pure Appl Chem. 2000;72.
- Jain AK, Singh P, Singh LP. Chelating ionophore based membrane sensors for copper (II) ions. Talanta. 2005;5:1355-61.
- Mascaró JJ, Ferrando J, Fontarnau R, et al. Green Hair. Cutis. 1995;56:37-40.
- Xiao F, Zhang X, Zhai H, et al. New halogenated disinfection byproducts in swimming pool water and their permeability across skin. Environ Sci Technol. 2012;46:7112-9.
- Yang WR, Chow E, Willett GD, et al. Exploring the use of the tripeptide Gly-Gly-His as a selective recognition element for the fabrication of electrochemical copper sensors. Analyst. 2003;128:712.
- Yoshimoto S, Mukai H, Kitano T, et al. Copper (II)-selective membrane electrode based on hydrotris(3-isopropylpyrazolyl)methane in a poly(vinyl chloride) matrix. Anal Chim Acta. 2003;494:207.