Original Article
, Volume: 12( 11)Biosynthesis of Nickel Nanoparticles Using Leaf Extract of Coriander
- *Correspondence:
- Pramod K, Department of Chemistry, Hutatma Rajguru College, Rajgurunagar, Pune, Maharashtra 410505, India, Tel: +91-9850658087; Fax: +91-2135-222-099; E-mail: pramodskulkarni3@gmail.com
Received: July 21, 2016; Accepted: August 02, 2016; Published: August 08, 2016
Citation: Vasudeo K, Pramod K. Biosynthesis of Nickel Nanoparticles Using Leaf Extract of Coriander. Biotechnol Ind J. 2016;12(11):106.
Abstract
Nickel nanoparticles has been synthesized using nickel chloride as a precursor and coriander leaf extract as a reducing agent and stabilizing agent at room temperature under ordinary condition. The progress of the reaction was monitored most probably observing change in colour of obtained solution. The synthesized nickel nanoparticle was confirmed by UV-Vis, FTIR and powder XRD techniques. The size of nanoparticle was determined by using powder XRD and found to be 30.71 nm. The merits of this method are easily available material and inexpensive starting material, short reaction time, easy to carry out.
Keywords
Biosynthesis; Coriander leaf; Nickel chloride; Nanoparticle; Nickel
Introduction
Synthesis of metal nanoparticles has gained significant interest in last twenty years because of their unusual properties and prospective applications in optical, electronic, catalytic, magnetic materials, thermal properties with corresponding bulk metals. In particular, magnetic nanomaterials show evidence of very large magnetoresistance, large coercivities, high curie temperatures and low saturation magnetization. Therefore, magnetic nanomaterial has attracted much attention in sensors, imaging, magnetic storage and ferro-fluids applications. In addition to magnetic properties, size-dependent melting properties of nickel nanoparticles also have been used to diffusion braze stainless steel at lower temperature and pressures [1].
A number of methods have been developed for the preparation of metal nanoparticles such as photo catalytic reduction, radiolytic reduction, sonochemical method, solvent extraction reduction, micro emulsion technique, polyol process and alcohol reduction [2]. Among the diverse kinds of metal nanoparticles, the preparation of some metal nanoparticles, such as nickel, copper and iron, are comparatively hard because they are easily oxidized. Nickel as an important transition metal, and nickel nanoparticles have wide ranging applications in the areas of permanent magnets, magnetic fluids, magnetic recording media, solar energy absorption, fuel cell electrodes, catalysts, biological activity [2]. Due to all these reasons the synthesis of Ni nanoparticles has attracted great focus of many scientists for its synthesis. Various methods were developed for the synthesis of nickel nanoparticles [3-18], many of these methods are accompanied by tedious process for isolation of nanoparticles, drastic reaction conditions, and use of expensive reagents, hence there is scope to develop new methods for synthesis of Ni nanoparticles.
The green synthesis as an eco-friendly pathway for nanoparticles synthesis. Here, we use coriander leaf extract for synthesis of Ni nanoparticles. Coriander (Coriandrum sativum L.) is spice crop which belongs to the family Apiaceae (Umbelliferae) is mainly cultivated from its seeds throughout the year [19]. A Constituent of coriander are phenolic acid including caffeic and chlorogenic acid and also contain essential oils like (E)-2-decenal, linalool, (E)-2-dodecenal, (E)-2-tetradecenal, 2-decen-1-ol, (E)-2-undecenal, dodecanal, (E)-2-tridecenal, (E)-2-hexadecenal, pentadecenal, and α-pinene [20]. The flavonoids include quercetin, keampferol, rhamnetin, apigenine and most of these compounds are known to suppress free radicals generated in the cell, when they are obtained through the diet [21]. Coriander has been reported to possess many pharmacological activities like antioxidant [22], antidiabetic [23], anti-mutagenic, anti-lipidemic, antispasmodic [24].
Materials and Methods
Preparation of coriander leaf extract
All the chemical and reagents used in this experiment were of analytical grade purchased from Loba Chemicals. The coriander leaves were purchased from Rajgurunagar vegetable markets, Pune, Maharashtra 410505, India. The coriander leaves were thoroughly washed and dried in shade. For preparing the plant broth solution, 20 gm dried leaves of coriander ware cut into small pieces and washed with distilled water. This was taken in a 250 ml beaker with 100 ml of distilled water and then boiled the mixture for 20 min at 80°C. The extract was filtered through Whatman filter No. 1 and then was stored at 5°C and can be used within a week.
Synthesis of Ni nanoparticles using coriander leaf extract
A volume of 10 ml of coriander leaf extract was added to 100 ml of 1 mm aqueous nickel chloride solution in a 250 ml Erlenmeyer flask. The colour of the solution changes from green to pale yellow after addition of coriander leaf extract and stirred the resulting solution for homogeneous mixing. The flask was kept at room temperature for overnight and Ni nanoparticles were separate out and settle at the bottom of mixed solution of nickel chloride and coriander leaf extract. The Ni nanoparticles thus obtained was purified by repeated centrifugation method at 5000 rpm for 15 min followed by re-dispersion of the pellet in deionized water. Then the Ni nanoparticles were dried in oven at 80°C. The progress of the reaction is shown in Figure 1.
Figure 1: Steps for synthesis of nickel nanoaprticle. a) Coriander leaves; b) Coriander leaves extract; c) Nickel chloride solution 0.1 M, coriander leaves extract, and nickel nanoparticles settled at bottom.
The pH of nickel chloride solution was 4.0, when we added coriander leaf extract to this solution, pH changes from 4.0 to 4.24. The pH of coriander leaf extract was 6.78. From this we confirmed that the capping between nickel and coriander leaf extract was taken place.
Equipment
UV-Vis spectral analysis was done by using a double beam spectrophotometer 2203 Systronics, in the range of 400 nm to 700 nm.
Fourier transform infrared spectrum was obtained using a JASCO FT/IR-6100 FT-IR spectrometer, at Department of Physics, Savitribai Phule Pune University, Pune, Maharashtra, India.
The XRD result was obtained using D8 advance diffractometer, Bruker AXS, at Department of Physics, Savitribai Phule Pune University, Pune, Maharashtra, India.
Results and Discussion
The synthesized Ni nanoparticles were characterized by UV-Vis, FTIR and XRD techniques.
UV-Vis spectroscopic analysis
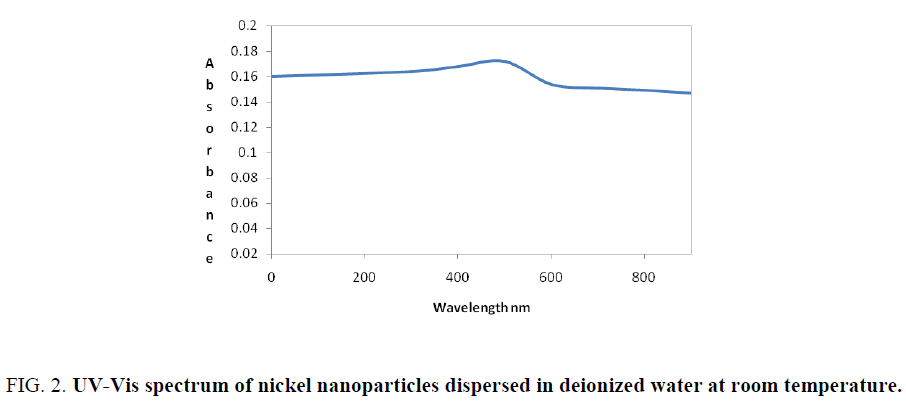
The reduction of nickel chloride to pure nickel nanoparticle was monitored by using ultraviolet visible spectrophotometer. The absorption spectrum of pale yellow nanoparticle solution prepared with the proposed method showed a palsmon absorption band with a maximum of 560 nm and the spectra is shown in Figure 2.
Fourier transform infrared spectroscopic analysis
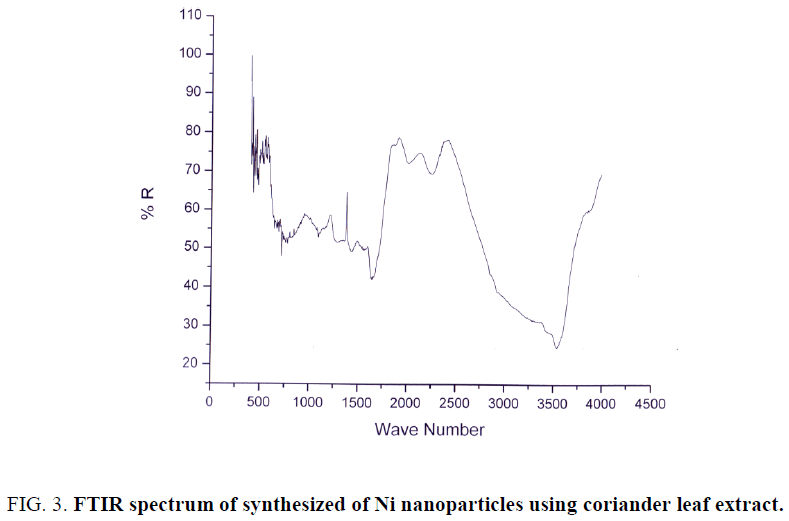
FTIR spectra of biosynthesized nickel nanoparticles were recorded to identify the capping and efficient stabilization of the metal nanoparticles by biomolecules present in coriander leaf extract. The FTIR spectrum of synthesized Ni nanoparticles using coriander leaf extract was shown in Figure 3. The band at 3495 cm-1 corresponds to O–H stretching of alcohols and phenols. The band at 1565 cm-1 corresponds to N–H bend of primary amines. The peak at 1413 cm-1 corresponds to C–N stretching of aromatic amino group. The band corresponds to 1674 cm-1 corresponds to carbonyl group of flavonoids, phenolic acids etc. The band at 1219 cm-1 corresponds to C–O linkages of phenol, acid, flavonoids. The band at 500 cm-1 corresponds to nickel. Therefore, the synthesized nickel nanoparticles were surrounded by proteins and metabolites such as phenolic acid, carboxylic acid, flavonoids. From the analysis of FTIR studies we confirmed that phenolic compounds have the stronger ability to bind metal indicating that the phenols could possibly form the metal nanoparticles to prevent agglomeration and thereby stabilized the medium. This suggests that the biological molecules could possibly perform dual functions of formation and stabilization of Ni nanoparticles in aqueous medium.
X-ray diffraction analysis
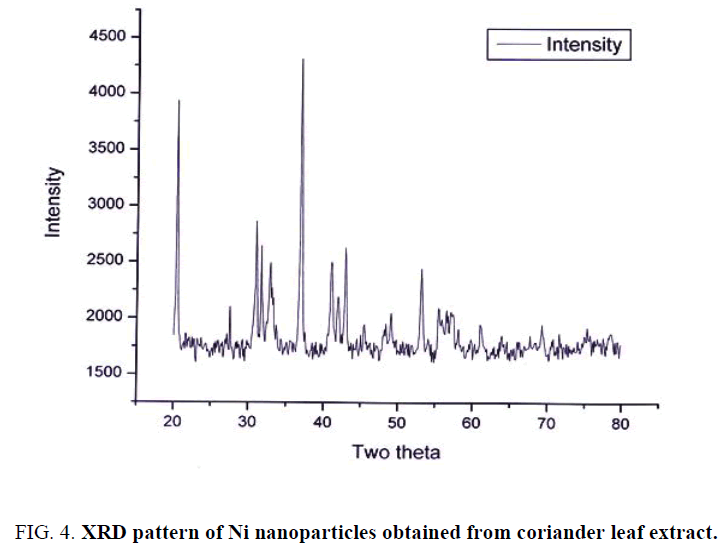
XRD pattern of synthesized Ni nanoparticles using coriander leaf broth extract is shown in Figure 4. The sample demonstrated a high crystallinity level with diffraction angles of 20.39°, 31.03°, 31.73°, 37.00°, 41.08°, 42.97° and 53.21° which correspond to the characteristic of face centered cubic of nickel lines indexed at (111), (211), (210) and (220). The diffraction angle observed at 20.39° which is related to the coriander leaf extract. The average size of the nickel nanoparticles was found to be 30.71 nm. The size of the NPs was determined using Debye-Scherrer equation, which may indicate a high surface area, and surface area to volume ration of the nanocrystals Table 1. The equation is written below:
| Peak No. | D in Å |
|---|---|
| 1 | 345.7738 |
| 2 | 293.575 |
| 3 | 443.52 |
| 4 | 271.8286 |
| 5 | 228.2608 |
| 6 | 302.8052 |
| 8 | 264.5543 |
Table 1. Calculated size of nickel nanoparticles for respective peak from XRD.
D=k × λ/β × cosθ
D=0.9 × 1.54/((π/180) × β × cosθ)
Where, D: grain size; K: constant (0.9 for spherical particle); Β: full width at half maxima of peaks; θ: corresponding angle for peaks.
Conclusion
References
- Eluri R, Paul B. Synthesis of nickel nanoparticles by hydrazine reduction: mechanistic study and continuous flow synthesis. J Nanopart Res. 2012;14(4):800-1.
- Chen D, Hsieh C. Synthesis of nickel nanoparticles in aqueous cationic surfactant solutions. J Mater Chem. 2002;12:2412-5.
- Zhang DE, Ni XM, Zheng HG, et al. Synthesis of needle-like nickel nanoparticles in water-in-oil microemulsion. Mater Lett. 2005;59:2011-4.
- Ying Z, Shengming J, Guanzhou Q, et al. Preparation of ultrafine nickel powder by polyol method and its oxidation product. MaterSciEngB. 2005;122:222-5.
- Wang H, Jiao X, Chen D. Monodispersed nickel nanoparticles with tunable phase and size: synthesis, characterization, and magnetic properties. J Phys Chem C. 2008;112(48):18793-7.
- Abu-Much R, Gedanken A. Sonochemical synthesis under a magnetic field: fabrication of nickel and cobalt particles and variation of their physical properties. Chemistry. 2008;14(32):10115-22.
- Gafner SL, Gafner YY. Analysis of gas-phase condensation of nickel nanoparticles. JExpTheoretPhy. 2008;107(4):712-22.
- Kim S, Kim B, Chung M, et al. The synthesis of nickel nanoparticles by liquid phase plasma processing. J Nanosci Nanotechnol. 2013;13(3):1997-2000.
- Xu W, Liew KY, Liu H, et al. Microwave-assisted synthesis of nickel nanoparticles. Mater Lett. 2008;62(17):2571-3.
- Eluri R, Paul B. Microwave assisted greener synthesis of nickel nanoparticles using sodium hypophosphite. Mater Lett. 2012;76:36-9.
- Wu SH, Chen DH. Synthesis and characterization of nickel nanoparticles by hydrazine reduction in ethylene glycol. JColloid Interface Sci. 2003;259(2):282-6.
- Capek I. Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv Colloid Interface Sci. 2004;110(1-2):49-74.
- Wang A, Yin H, Ren M, et al. Preparation of nickel nanoparticles with different sizes and structures and catalytic activity in the hydrogenation of p-nitrophenol. New J Chem. 2010;34:708-13.
- Singh K, Kate KH, Chilukuri VV, et al. Glycerol mediated low temperature synthesis of nickel nanoparticles by solution reduction method. J Nanosci Nanotechnol. 2011;11(6):5131-6.
- Cordente N, Respaud M, Sencoq F, et al. Synthesis and magnetic properties of nickel nanorods. Nano Lett. 2001;1(10):565-8.
- Park J, Kang E, Son S, et al. Monodisperse nanoparticles of Ni and Nio: synthesis, characterization, self-assembled superlattices, and catalytic applications in the suzuki coupling reaction. AdvMater. 2005;17(4):429-34.
- Babu RS, Prabhu P, Narayanan SS. Green synthesized nickel nanoparticles modified electrode in ionic liquid medium and its application towards determination of biomolecules. Talanta. 2013;110:135-43.
- Kobayashi K, Koshio A, Kokai F. Laser vaporization synthesis of carbon-encapsulated Ni nanoparticles and hollow carbon shells from mixture of perylenetetracarboxylic dianhydride and NiCl2.6H2O. New Diamond FrontCarbon Technol. 2005;15:83-9.
- Bhat S, Kaushal P, Kaur M, et al. Coriander (Coriandrum sativum L.): Processing, nutritional and functional aspects. Afr J Plant Sci. 2014;8(1):25-33.
- Shahwar MK, El-Ghorab AH, Anjum FM, et al. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: volatile and non-volatile extracts. IntJFood Prop. 2012;15(4):736-47.
- Rajeshwari U, Andallu B. Medicinal benefits of coriander (Coriandrum Sativum L). Spatula DD. 2011;1(1):51-8.
- Hwang E, Lee D, Park SH, et al. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type i and MMP-1 expression. J Med Food. 2014;17(9):985-95.
- Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant Coriandrum sativum (coriander). Br J Nutr. 1999;81(3):203-9.
- Pathak NL, Kasture SB, Bhatt NM, et al. Phytopharmacological properties of coriander sativumas a potential medicinal tree: An overview. JApplPharmaSci. 2011;01(04):20-5.